Identification of phosphorylation-induced changes in vimentin intermediate filaments by site-directed spin labeling and electron paramagnetic resonance
- PMID: 18803396
- PMCID: PMC2656440
- DOI: 10.1021/bi801137m
Identification of phosphorylation-induced changes in vimentin intermediate filaments by site-directed spin labeling and electron paramagnetic resonance
Abstract
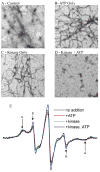
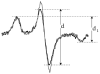
Phosphorylation drives the disassembly of the vimentin intermediate filament (IF) cytoskeleton at mitosis. Chromatographic analysis has suggested that phosphorylation produces a soluble vimentin tetramer, but little has been determined about the structural changes that are caused by phosphorylation or the structure of the resulting tetramer. In this study, site-directed spin labeling and electron paramagnetic resonance (SDSL-EPR) were used to examine the structural changes resulting from protein kinase A phosphorylation of vimentin IFs in vitro. EPR spectra suggest that the tetrameric species resulting from phosphorylation is the A11 configuration. EPR spectra also establish that the greatest degree of structural change was found in the linker 2 and the C-terminal half of the rod domain, despite the fact that most phosphorylation occurs in the N-terminal head domain. The phosphorylation-induced changes notably affected the proposed "trigger sequences" located in the linker 2 region, which have been hypothesized to mediate the induction of coiled-coil formation. These data are the first to document specific changes in IF structure resulting from a physiologic regulatory mechanism and provide further evidence, also generated by SDSL-EPR, that the linker regions play a key role in IF structure and regulation of assembly/disassembly.
Figures


References
-
- Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci. 2001;114:2569–2575. - PubMed
-
- Albers K, Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–279. - PubMed
-
- Hanukoglu I, Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983;33:915–924. - PubMed
-
- Steinert PM, Steven AC, Roop DR. The molecular biology of intermediate filaments. Cell. 1985;42:411–420. - PubMed
-
- Conway J, Parry DD. Intermediate filament structure 3. Int J Biol Macromol. 1988;10
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

