A retrospective database study comparing treatment outcomes and cost associated with choice of fixed-dose inhaled corticosteroid/long-acting beta-agonists for asthma maintenance treatment in Germany
- PMID: 18803555
- PMCID: PMC2680329
- DOI: 10.1111/j.1742-1241.2008.01895.x
A retrospective database study comparing treatment outcomes and cost associated with choice of fixed-dose inhaled corticosteroid/long-acting beta-agonists for asthma maintenance treatment in Germany
Abstract
Aims: This retrospective, observational cohort study aimed to compare treatment outcomes and healthcare costs in the year after initiation of maintenance treatment with budesonide/formoterol or salmeterol/fluticasone in a German healthcare setting.
Methods: Data on German asthma patients initiating treatment with budesonide/formoterol or salmeterol/fluticasone between June 2001 and June 2005 were obtained from the IMS Disease Analyzer database. The primary outcome was the probability of treatment success, defined according to short-acting beta(2)-agonist prescriptions and switches or addition of controller medications, during the postindex year. A secondary definition of treatment success included hospitalisations and oral corticosteroid (OCS) prescriptions. Secondary outcomes included severe asthma exacerbations, defined as >or=1 OCS prescription, asthma-related hospitalisation and/or referral. The effect of treatment on costs was estimated using generalised linear models, adjusting for patient and physician characteristics.
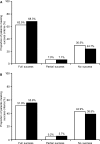
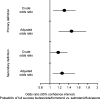
Results: There were no significant differences between the budesonide/formoterol (n = 1456) and salmeterol/fluticasone (n = 982) groups in disease severity markers in the pre-index year. Patients on budesonide/formoterol had a 44% greater probability of treatment success [odds ratio (OR): 1.44; p = 0.0003] according to the primary definition and a 26% greater probability (OR: 1.26; p = 0.0119) according to the secondary definition, fewer severe exacerbations (-33.4%; p = 0.0123) and fewer OCS prescriptions (-31.5%; p = 0.0082) compared with salmeterol/fluticasone, after controlling for baseline characteristics. Adjusting for covariates, budesonide/formoterol had a significant inverse relationship on asthma-related costs compared with salmeterol/fluticasone (-13.4%; p < 0.001). Total cost (asthma- and non-asthma-related costs) was 12.6% lower for budesonide/formoterol (p < 0.0001).
Conclusion: This study suggests that for patients with chronic asthma in Germany, budesonide/formoterol rather than salmeterol/fluticasone had a higher likelihood of treatment success, and that budesonide/formoterol is the less costly option. Although the cohorts appeared to be well matched at baseline, the results should be interpreted with caution given the observational nature of the study.
Figures

Similar articles
-
Inhaled corticosteroids or long-acting beta-agonists alone or in fixed-dose combinations in asthma treatment: a systematic review of fluticasone/budesonide and formoterol/salmeterol.Clin Ther. 2009 Dec;31(12):2779-803. doi: 10.1016/j.clinthera.2009.12.021. Clin Ther. 2009. PMID: 20110019
-
Treatment comparison of budesonide/formoterol with salmeterol/fluticasone propionate in adults aged > or =16 years with asthma: post hoc analysis of a randomized, double-blind study.Clin Drug Investig. 2010;30(9):565-79. doi: 10.2165/11533450-000000000-00000. Clin Drug Investig. 2010. PMID: 20593912 Clinical Trial.
-
Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma.Curr Med Res Opin. 2004;20(2):225-40. doi: 10.1185/030079903125002928. Curr Med Res Opin. 2004. PMID: 15006018 Clinical Trial.
-
Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study.Clin Drug Investig. 2012 Jul 1;32(7):439-49. doi: 10.2165/11598840-000000000-00000. Clin Drug Investig. 2012. PMID: 22607479 Clinical Trial.
-
Canadian economic evaluation of budesonide-formoterol as maintenance and reliever treatment in patients with moderate to severe asthma.Can Respir J. 2007 Jul-Aug;14(5):269-75. doi: 10.1155/2007/560819. Can Respir J. 2007. PMID: 17703241 Free PMC article. Review.
Cited by
-
Comparative analysis of budesonide/formoterol and fluticasone/salmeterol combinations in COPD patients: findings from a real-world analysis in an Italian setting.Int J Chron Obstruct Pulmon Dis. 2016 Nov 4;11:2749-2755. doi: 10.2147/COPD.S114554. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27853362 Free PMC article.
-
Management of asthma and chronic obstructive pulmonary disease with combination inhaled corticosteroids and long-acting β-agonists: a review of comparative effectiveness research.Drugs. 2014 May;74(7):737-55. doi: 10.1007/s40265-014-0214-8. Drugs. 2014. PMID: 24797158 Free PMC article. Review.
References
-
- British Thoracic Society, Scottish Intercollegiate Guidelines Network . British Guideline on the Management of Asthma, a National Clinical Guideline. [accessed June 2008]. http://www.brit-thoracic.org.uk/
-
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. [accessed April 2008]. http://www.ginasthma.com.
-
- Desfougeres J-L, Sohier B, Freedman D, et al. Has asthma control improved since AIRE? Results of a survey in 5 European countries. Eur Respir J. 2007;30:249s.
-
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–44. - PubMed
-
- Gibson PG, Powell H, Ducharme FM. Differential effects of maintenance long-acting beta-agonist and inhaled corticosteroid on asthma control and asthma exacerbations. J Allergy Clin Immunol. 2007;119:344–50. - PubMed

