Metformin suppresses intestinal polyp growth in ApcMin/+ mice
- PMID: 18803638
- PMCID: PMC11159964
- DOI: 10.1111/j.1349-7006.2008.00933.x
Metformin suppresses intestinal polyp growth in ApcMin/+ mice
Abstract
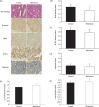
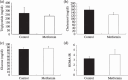
Metformin is a biguanide derivative that is widely used in the treatment of diabetes mellitus. One of the pharmacological targets of metformin is adenosine monophosphate-activated protein kinase (AMPK). We investigated the effect of metformin on the suppression of intestinal polyp formation in Apc(Min/+) mice. Administration of metformin (250 mg/kg) did not reduce the total number of intestinal polyp formations, but significantly reduced the number of intestinal polyp formations larger than 2 mm in diameter in Apc(Min/+) mice. To examine the indirect effect of metformin, the index of insulin resistance and serum lipid levels in Apc(Min/+) mice were assessed. These factors were not significantly attenuated by the treatment with metformin, indicating that the suppression of polyp growth is not due to the indirect drug action. The levels of tumor cell proliferation as determined by 5-bromodeoxyuridine and proliferating cell nuclear antigen immunohistochemical staining, and apoptosis, via transferase deoxytidyl uridine end labeling staining, in the polyps of metformin-treated mice were not significantly different in comparison to those of control mice. Gene expression of cyclin D1 and c-myc in intestinal polyps were also not significantly different between those two groups. In contrast, metformin activated AMPK in the intestinal polyps, resulting in the inhibition of the activation of mammalian target of rapamycin, which play important roles in the protein synthesis machinery. Metformin suppressed the polyp growth in Apc(Min/+) mice, suggesting that it may be a novel candidate as a chemopreventive agent for colorectal cancer.
Figures



References
-
- Jemal A, Tiwari RC, Murray T et al . American Cancer Society. Cancer statistics, 2004. CA Cancer J Clin 2004; 54: 8–29. - PubMed
-
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoclassic in the mouse. Science 1990; 247: 322–4. - PubMed
-
- Su LK, Kinzler KW, Vogelstein B et al . Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992; 256: 668–70. - PubMed
-
- He TC, Sparks AB, Rago C et al . Identification of c‐MYC as a target of the APC pathway. Science 1998; 281: 1509–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

