Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells
- PMID: 18803764
- PMCID: PMC2527370
- DOI: 10.1111/j.1365-2249.2008.03726.x
Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells
Abstract
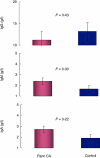
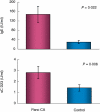
In addition to allergy and parasitic infections, immunoglobulin E (IgE) has been shown recently to possess anti-viral and anti-cancer effects. We investigated serum levels of IgE, its low-affinity receptor, soluble CD23 (sCD23) in patients with pancreatic cancer and the effect of IgE against pancreatic cancer cells. Twelve patients were evaluated for pancreatic cancer by imaging and confirmed by biopsy. Fifteen healthy volunteers served as controls. Serum Igs (IgG, IgM, IgA, IgE) and sCD23 levels were determined (enzyme-linked immunosorbent assay, nephelometry) and the presence of cancer-specific IgE was assessed (fluorescence microscopy, Western blot). IgE anti-cancer activity was determined by antibody-dependent cell-mediated cytotoxicity (ADCC). Serum levels of IgE and sCD23 were elevated significantly in patients with pancreatic cancer versus controls, whereas no differences were observed in other Ig isotypes (IgG, IgM, IgA). Flow cytometry and immunofluorescence microscopy demonstrated similar presence of IgG and IgE pancreatic cancer Igs. However, Western blot analysis indicated differences in IgG and IgE antigen-specific antibodies; IgE antibody recognized a 50 kD protein. ADCC studies demonstrated that serum and purified IgE-mediated cytotoxicity against pancreatic cancer cells, effects which were reversed with anti-IgE neutralizing antibody and IgE depletion (immunoaffinity); greater cytotoxicity was observed in patient serum when compared with healthy controls. These data suggest that IgE and sCD23 may serve as useful biomarkers for patients with pancreatic cancer and may be important in the immune response to this disease in that IgE-directed therapy may help to direct treatment.
Figures



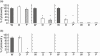
Similar articles
-
IgE anti-Borrelia burgdorferi components (p18, p31, p34, p41, p45, p60) and increased blood CD8+CD60+ T cells in children with Lyme disease.Scand J Immunol. 2007 Apr;65(4):376-82. doi: 10.1111/j.1365-3083.2007.01904.x. Scand J Immunol. 2007. PMID: 17386029
-
Elevated expression of CD23 on peripheral blood B lymphocytes from patients with bullous pemphigoid: correlation with increased serum IgE.J Dermatol Sci. 2004 Jun;35(1):53-9. doi: 10.1016/j.jdermsci.2004.03.009. J Dermatol Sci. 2004. PMID: 15194147
-
[Investigation of immunoglobulin isotypes in patients with fasciolosis].Mikrobiyol Bul. 2009 Jul;43(3):471-5. Mikrobiyol Bul. 2009. PMID: 19795623 Turkish.
-
Antibodies as biomarkers for cancer risk: a systematic review.Clin Exp Immunol. 2022 Jul 22;209(1):46-63. doi: 10.1093/cei/uxac030. Clin Exp Immunol. 2022. PMID: 35380164 Free PMC article.
-
Immunoglobulin Glycosylation Effects in Allergy and Immunity.Curr Allergy Asthma Rep. 2016 Nov;16(11):79. doi: 10.1007/s11882-016-0658-x. Curr Allergy Asthma Rep. 2016. PMID: 27796794 Review.
Cited by
-
IgE immunotherapy against cancer.Curr Top Microbiol Immunol. 2015;388:109-49. doi: 10.1007/978-3-319-13725-4_6. Curr Top Microbiol Immunol. 2015. PMID: 25553797 Free PMC article. Review.
-
IgE-Based Therapeutic Combination Enhances Antitumor Response in Preclinical Models of Pancreatic Cancer.Mol Cancer Ther. 2021 Dec;20(12):2457-2468. doi: 10.1158/1535-7163.MCT-21-0368. Epub 2021 Oct 8. Mol Cancer Ther. 2021. PMID: 34625505 Free PMC article.
-
Pancreatic Cancer: A Review of Risk Factors.Life (Basel). 2024 Aug 5;14(8):980. doi: 10.3390/life14080980. Life (Basel). 2024. PMID: 39202722 Free PMC article. Review.
-
The causal effect of atopic dermatitis on lung cancer: A Mendelian randomization study.Skin Res Technol. 2024 Jul;30(7):e13841. doi: 10.1111/srt.13841. Skin Res Technol. 2024. PMID: 38965791 Free PMC article.
-
Harnessing the Anti-Tumor Mediators in Mast Cells as a New Strategy for Adoptive Cell Transfer for Cancer.Front Oncol. 2022 Mar 31;12:830199. doi: 10.3389/fonc.2022.830199. eCollection 2022. Front Oncol. 2022. PMID: 35433433 Free PMC article. Review.
References
-
- Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population-based study. Am J Gastroenterol. 2007;102:1377–82. - PubMed
-
- Saif MW. Pancreatic cancer: are we moving forward yet? Highlights from the Gastrointestinal Cancers Symposium. Orlando, FL, USA, 20 January. J Pancreas. 2007;8:166–76. - PubMed
-
- Zamai M, VandeVen M, Farao M, et al. Camptothecin poly[n-(2-hydroxypropyl) methacrylamide] copolymers in antitopoisomerase-I tumor therapy: intratumor release and antitumor efficacy. Mol Cancer Ther. 2003;2:29–40. - PubMed
-
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

