Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders
- PMID: 18803808
- PMCID: PMC2562999
- DOI: 10.1186/1756-6606-1-6
Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders
Abstract
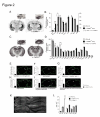
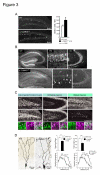
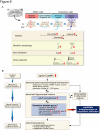
Elucidating the neural and genetic factors underlying psychiatric illness is hampered by current methods of clinical diagnosis. The identification and investigation of clinical endophenotypes may be one solution, but represents a considerable challenge in human subjects. Here we report that mice heterozygous for a null mutation of the alpha-isoform of calcium/calmodulin-dependent protein kinase II (alpha-CaMKII+/-) have profoundly dysregulated behaviours and impaired neuronal development in the dentate gyrus (DG). The behavioral abnormalities include a severe working memory deficit and an exaggerated infradian rhythm, which are similar to symptoms seen in schizophrenia, bipolar mood disorder and other psychiatric disorders. Transcriptome analysis of the hippocampus of these mutants revealed that the expression levels of more than 2000 genes were significantly changed. Strikingly, among the 20 most downregulated genes, 5 had highly selective expression in the DG. Whereas BrdU incorporated cells in the mutant mouse DG was increased by more than 50 percent, the number of mature neurons in the DG was dramatically decreased. Morphological and physiological features of the DG neurons in the mutants were strikingly similar to those of immature DG neurons in normal rodents. Moreover, c-Fos expression in the DG after electric footshock was almost completely and selectively abolished in the mutants. Statistical clustering of human post-mortem brains using 10 genes differentially-expressed in the mutant mice were used to classify individuals into two clusters, one of which contained 16 of 18 schizophrenic patients. Nearly half of the differentially-expressed probes in the schizophrenia-enriched cluster encoded genes that are involved in neurogenesis or in neuronal migration/maturation, including calbindin, a marker for mature DG neurons. Based on these results, we propose that an "immature DG" in adulthood might induce alterations in behavior and serve as a promising candidate endophenotype of schizophrenia and other human psychiatric disorders.
Figures

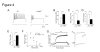

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

