Excitatory actions of peptide histidine isoleucine on thalamic relay neurons
- PMID: 18804119
- PMCID: PMC2645485
- DOI: 10.1016/j.neuropharm.2008.08.028
Excitatory actions of peptide histidine isoleucine on thalamic relay neurons
Abstract
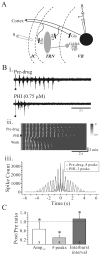
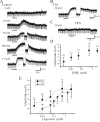
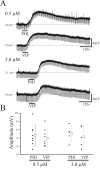
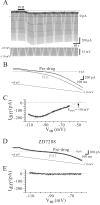
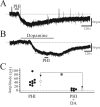
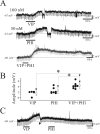
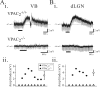
Peptide histidine isoleucine (PHI) and vasoactive intestinal peptide (VIP) are neuropeptides synthesized from a common precursor, prepro-VIP, and share structural similarity and biological functions in many systems. Within the central nervous system and peripheral tissues, PHI and VIP have overlapping distribution. PHI-mediated functions are generally via activation of VIP receptors; however, the potency and affinity of PHI for VIP receptors are significantly lower than VIP. In addition, several studies suggest distinct PHI receptors that are independent of VIP receptors. PHI receptors have been cloned and characterized in fish, but their existence in mammals is still unknown. This study focuses on the functional role of PHI in the thalamus because of the localization of both PHI and VIP receptors in this brain region. Using extracellular multiple-unit recording techniques, we found that PHI strongly attenuated the slow intrathalamic rhythmic activity. Using intracellular recording techniques, we found that PHI selectively depolarized thalamic relay neurons via an enhancement of the hyperpolarization-activated mixed cation current, Ih. Further, the actions of PHI were occluded by VIP and dopamine, indicating these modulators converge onto a common mechanism. In contrast to previous work, we found that PHI was more potent than VIP in producing excitatory actions on thalamic neurons. We next used the transgenic mice lacking a specific VIP receptor, VPAC2, to identify its possible role in PHI-mediated actions in the thalamus. PHI depolarized all relay neurons tested from wild-type mice (VPAC2(+/+)); however, in knockout mice (VPAC2(-/-)), PHI produced no change in membrane potential in all neurons tested. Our findings indicate that excitatory actions of PHI are mediated by VPAC2 receptors, not by its own PHI receptors and the excitatory actions of PHI clearly attenuate intrathalamic rhythmic activities, and likely influence information transfer through thalamocortical circuits.
Figures
Similar articles
-
Excitatory actions of vasoactive intestinal peptide on mouse thalamocortical neurons are mediated by VPAC2 receptors.J Neurophysiol. 2006 Aug;96(2):858-71. doi: 10.1152/jn.01115.2005. Epub 2006 Apr 26. J Neurophysiol. 2006. PMID: 16641377
-
Vasoactive intestinal peptide selectively depolarizes thalamic relay neurons and attenuates intrathalamic rhythmic activity.J Neurophysiol. 2003 Aug;90(2):1224-34. doi: 10.1152/jn.00280.2003. Epub 2003 Apr 23. J Neurophysiol. 2003. PMID: 12711712
-
VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice.J Pharmacol Exp Ther. 2005 Aug;314(2):745-52. doi: 10.1124/jpet.105.086405. Epub 2005 May 4. J Pharmacol Exp Ther. 2005. PMID: 15872042
-
[Peptide histidine-isoleucine and and its human analogue peptide histidine-methionine: localization, receptors and biological function].Postepy Hig Med Dosw (Online). 2004 Feb 26;58:18-26. Postepy Hig Med Dosw (Online). 2004. PMID: 15069375 Review. Polish.
-
Neuroprotective potential of three neuropeptides PACAP, VIP and PHI.Pharmacol Rep. 2005 May-Jun;57(3):307-20. Pharmacol Rep. 2005. PMID: 15985713 Review.
Cited by
-
Frequency-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus.J Neurophysiol. 2010 Sep;104(3):1758-67. doi: 10.1152/jn.00010.2010. Epub 2010 Jul 21. J Neurophysiol. 2010. PMID: 20660425 Free PMC article.
References
-
- Anagnostides AA, Manolas K, Christofides ND, Yiangou Y, Welbourn RB, Bloom SR, Chadwick VS. Peptide histidine isoleucine (PHI). A secretagogue in porcine intestine. Dig Dis Sci. 1983;28:893–896. - PubMed
-
- Bailey CJ, Wilkes LC, Conlon JM, Armstrong PH, Buchanan KD. Effects of gastric inhibitory polypeptide, vasoactive intestinal polypeptide and peptide histidine isoleucine on the secretion of hormones by isolated mouse pancreatic islets. J Endocrinol. 1990;125:375–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

