A subcortical maternal complex essential for preimplantation mouse embryogenesis
- PMID: 18804437
- PMCID: PMC2597058
- DOI: 10.1016/j.devcel.2008.07.010
A subcortical maternal complex essential for preimplantation mouse embryogenesis
Abstract
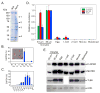
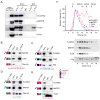
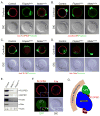
We have identified a subcortical maternal complex (SCMC) that assembles during oocyte growth and is essential for zygotes to progress beyond the first embryonic cell divisions. At least four maternally encoded proteins contribute to this MDa complex: FLOPED, MATER, and TLE6 interact with each other while Filia binds independently to MATER. Although the transcripts encoding these proteins are degraded during meiotic maturation and ovulation, the SCMC proteins persist in the early embryo. The SCMC, located in the subcortex of eggs, is excluded from regions of cell-cell contact in the cleavage-stage embryo and segregates to the outer cells of the morulae and blastocyst. Floped(tm/tm) and/or Mater(tm/tm) eggs lack the SCMC but can be fertilized. However, these embryos do not progress beyond cleavage stage development and female mice are sterile. The proteins are conserved in humans, and similar maternal effect mutations may result in recurrent embryonic loss.
Figures



References
-
- Aronson J, Solter D. Developmental potency of gametic and embryonic genomes revealed by nuclear transfer. Curr Top Dev Biol. 1987;23:55–71. - PubMed
-
- Bajoghli B. Evolution of the Groucho/Tle gene family: gene organization and duplication events. Dev Genes Evol. 2007;217:613–618. - PubMed
-
- Bolton VN, Oades PJ, Johnson MH. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984;79:139–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

