The underestimated role of olfaction in avian reproduction?
- PMID: 18804490
- PMCID: PMC2692081
- DOI: 10.1016/j.bbr.2008.08.036
The underestimated role of olfaction in avian reproduction?
Abstract
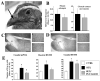
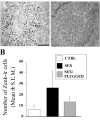
Until the second half of the 20th century, it was broadly accepted that most birds are microsmatic if not anosmic and unable to detect and use olfactory information. Exceptions were eventually conceded for species like procellariiforms, vultures or kiwis that detect their food at least in part based on olfactory signals. During the past 20-30 years, many publications have appeared indicating that this view is definitely erroneous. We briefly review here anatomical, electrophysiological and behavioral data demonstrating that birds in general possess a functional olfactory system and are able to use olfactory information in a variety of ethological contexts, including reproduction. Recent work also indicates that brain activation induced by sexual interactions with a female is significantly affected by olfactory deprivation in Japanese quail. Brain activation was measured via immunocytochemical detection of the protein product of the immediate early gene c-fos. Changes observed concerned two brain areas that play a key role in the control of male sexual behavior, the medial preoptic nucleus and the bed nucleus of the stria terminalis therefore suggesting a potential role of olfaction in the control of reproduction. The widespread idea that birds are anosmic or microsmatic is thus not supported by the available experimental data and presumably originates in our anthropomorphic view that leads us to think that birds do not smell because they have a rigid beak and nostrils and do not obviously sniff. Experimental analysis of this phenomenon is thus warranted and should lead to a significant change in our understanding of avian biology.
Figures
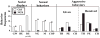
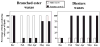
References
-
- Amo L, et al. Predator odour recognition and avoidance in a songbird. Functional Ecology. 2008;22:289–293.
-
- Ball GF, Tlemçani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2970. - PubMed
-
- Balthazart J, Schoffeniels E. Pheromones are involved in the control of sexual behaviour in birds. Naturwissenschaften. 1979;66:55–56. - PubMed
-
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

