Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene
- PMID: 18804506
- PMCID: PMC2629409
- DOI: 10.1016/j.vaccine.2008.08.069
Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene
Abstract
The type of immune response induced by a vaccine is a critical factor that determines its effectiveness in preventing infection or disease. Inactivated and live rabies virus (RV) vaccine strains elicit an IgG1-biased and IgG1/IgG2a-balanced antibody response, respectively. However, IgG2a antibodies are potent inducers of anti-viral effector functions, and therefore, a viral vaccine vector that can elicit an IgG2a-biased antibody response may be more effective against RV infection. Here we describe the humoral immune response of a live replication-deficient phosphoprotein (P)-deleted RV vector (SPBN-DeltaP), or a recombinant P-deleted virus that expresses two copies of the RV glycoprotein (G) gene (SPBN-DeltaP-RVG), and compare it to a UV-inactivated RV. Mice inoculated with UV-inactivated RV induced predominantly an IgG1-specific antibody response, while live recombinant SPBN-DeltaP exhibited a mixed IgG1/IgG2a antibody response, which is consistent with the isotype profiles from the replication-competent parental viruses. Survivorship in mice after pathogenic RV challenge indicates a 10-fold higher efficiency of live SPBN-DeltaP compared to UV-inactivated SPBN-DeltaP. In addition, SPBN-DeltaP-RVG induced a more rapid and robust IgG2a response that protected mice more effectively than SPBN-DeltaP. Of note, 10(3)ffu of SPBN-DeltaP-RVG-induced anti-RV antibodies that were 100% protective in mice against pathogenic RV challenge. The increased immune response was directed not only against RV G but also against the ribonucleoprotein (RNP), indicating that the expression of two RV G genes from SPBN-DeltaP-RVG enhances the immune response to other RV antigens as well. In addition, Rag2 mice inoculated intramuscularly with 10(5)ffu/mouse of SPBN-DeltaP showed no clinical signs of rabies, and no viral RNA was detected in the spinal cord or brain of inoculated mice. Therefore, the safety of the P-deleted vectors along with the onset and magnitude of the IgG2a-induced immune response by SPBN-DeltaP-RVG indicate that this vector holds great promise as either a therapeutic or preventative vaccine against RV or other infectious diseases.
Figures



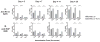


References
-
- McKenna PM, Koser ML, Carlson KR, et al. Highly attenuated rabies virus-based vaccine vectors expressing simian-human immunodeficiency virus89.6P Env and simian immunodeficiency virusmac239 Gag are safe in rhesus macaques and protect from an AIDS-like disease. Journal of Infectious Diseases. 2007;195(7):980–8. - PubMed
-
- Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175(2):485–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

