Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial
- PMID: 18805335
- PMCID: PMC2610850
- DOI: 10.1016/S0140-6736(08)61448-8
Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial
Abstract
Background: Preliminary evidence is equivocal about the role of exhaled nitric oxide (NO) in clinical asthma management. We aimed to assess whether measurement of exhaled NO, as a biomarker of airway inflammation, could increase the effectiveness of asthma treatment, when used as an adjunct to clinical care based on asthma guidelines for inner-city adolescents and young adults.
Methods: We did a randomised, double-blind, parallel-group trial at ten centres in the USA. We screened 780 inner-city patients, aged 12-20 years, who had persistent asthma. All patients completed a run-in period of 3 weeks on a regimen based on standard treatment. 546 eligible participants who adhered to treatment during this run-in period were then randomly assigned to 46 weeks of either standard treatment, based on the guidelines of the National Asthma Education and Prevention Program (NAEPP), or standard treatment modified on the basis of measurements of fraction of exhaled NO. The primary outcome was the number of days with asthma symptoms. We analysed patients on an intention-to-treat basis. This trial is registered with clinicaltrials.gov, number NCT00114413.
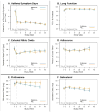
Findings: During the 46-week treatment period, the mean number of days with asthma symptoms did not differ between the treatment groups (1.93 [95% CI 1.74 to 2.11] in the NO monitoring group vs 1.89 [1.71 to 2.07] in the control group; difference 0.04 [-0.22 to 0.29], p=0.780). Other symptoms, pulmonary function, and asthma exacerbations did not differ between groups. Patients in the NO monitoring group received higher doses of inhaled corticosteroids (difference 119 mug per day, 95% CI 49 to 189, p=0.001) than controls. Adverse events did not differ between treatment groups (p>0.1 for all adverse events).
Interpretation: Conventional asthma management resulted in good control of symptoms in most participants. The addition of fraction of exhaled NO as an indicator of control of asthma resulted in higher doses of inhaled corticosteroids, without clinically important improvements in symptomatic asthma control.
Figures
Comment in
-
Exhaled nitric oxide in guideline-based asthma management.Lancet. 2008 Sep 20;372(9643):1015-7. doi: 10.1016/S0140-6736(08)61420-8. Lancet. 2008. PMID: 18805314 No abstract available.
-
Clinical use of exhaled nitric oxide measurements.Lancet. 2009 Jan 31;373(9661):382; author reply 382. doi: 10.1016/S0140-6736(09)60134-3. Lancet. 2009. PMID: 19186272 No abstract available.
References
-
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. Report No.: Publication no. 08-4051.1.
-
- Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–138. - PubMed
-
- Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, Szefler SJ. The Aerocrine exhaled nitric oxide monitoring system Niox is cleared by the US food and drug administration for monitoring therapy in asthma. J Allergy Clin Immunol. 2004;114(5):1241–56. - PubMed
-
- Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995;152(2):800–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

