Nitric oxide-donating aspirin inhibits the growth of pancreatic cancer cells through redox-dependent signaling
- PMID: 18805632
- PMCID: PMC2649683
- DOI: 10.1016/j.canlet.2008.08.006
Nitric oxide-donating aspirin inhibits the growth of pancreatic cancer cells through redox-dependent signaling
Abstract
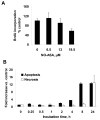
The novel chemopreventive nitric oxide-donating aspirin (NO-ASA) prevents nearly 90% of ductal adenocarcinomas in a animal tumor model. To decipher the mechanism of this effect, we studied in BxPC-3 human pancreatic cancer cells the sequence of signaling events leading from NO-ASA treatment to cell growth inhibition. NO-ASA inhibited the growth of BxPC-3 cells (IC(50) =13 microM), by inhibiting proliferation modestly and inducing apoptosis, necrosis and G(1)/S cell cycle block. At 15 min of treatment with NO-ASA, the intracellular levels of reactive oxygen species (ROS) began increasing (peak at 8h, baseline levels by 24h). ROS activated almost immediately in a time- and concentration-dependent manner the MAPK pathways p38, ERK and JNK (their activation was abrogated by the antioxidant N-acetylcysteine). MAPK activation induced p21(cip-1), which suppressed the levels of cyclin D1 that controls the G(1)/S cell cycle transition. NO-ASA induced COX-2 expression starting 90 min after p21(cip-1) was induced. When COX-2 expression was knocked down using siRNA against cox-2, the expression of p21(cip-1) was induced by NO-ASA, regardless of the level of expression of COX-2, suggesting a marginal, if any, role for COX-2 in the growth inhibitory effect of NO-ASA. These findings along with the temporal sequence of individual changes indicate a signaling sequence that involves ROS-->MAPKs-->p21(cip-1)-->cyclin D1-->cell death. Our findings establish the critical role of ROS as proximal signaling molecules in the action of anticancer compounds and may be useful in designing mechanism-driven approaches to cancer control.
Figures






Similar articles
-
NOSH-aspirin (NBS-1120) inhibits estrogen receptor-negative breast cancer in vitro and in vivo by modulating redox-sensitive signaling pathways.J Pharmacol Exp Ther. 2025 Jan;392(1):100019. doi: 10.1124/jpet.124.002240. Epub 2024 Nov 22. J Pharmacol Exp Ther. 2025. PMID: 39892987
-
Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect.Carcinogenesis. 2009 Mar;30(3):512-9. doi: 10.1093/carcin/bgp015. Epub 2009 Jan 9. Carcinogenesis. 2009. PMID: 19136474 Free PMC article.
-
NO-releasing aspirin exerts stronger growth inhibitory effect on Barrett's adenocarcinoma cells than traditional aspirin.J Physiol Pharmacol. 2006 Dec;57 Suppl 12:15-24. J Physiol Pharmacol. 2006. PMID: 17244951
-
Molecular targets of nitric-oxide-donating aspirin in cancer.Biochem Soc Trans. 2005 Aug;33(Pt 4):701-4. doi: 10.1042/BST0330701. Biochem Soc Trans. 2005. PMID: 16042578 Review.
-
Novel agents for cancer prevention based on nitric oxide.Biochem Soc Trans. 2007 Nov;35(Pt 5):1364-8. doi: 10.1042/BST0351364. Biochem Soc Trans. 2007. PMID: 17956352 Review.
Cited by
-
Urinary prostaglandin E2 metabolite and pancreatic cancer risk: case-control study in urban Shanghai.PLoS One. 2015 Feb 13;10(2):e0118004. doi: 10.1371/journal.pone.0118004. eCollection 2015. PLoS One. 2015. PMID: 25679523 Free PMC article.
-
The differential cell signaling effects of two positional isomers of the anticancer NO-donating aspirin.Int J Oncol. 2009 Oct;35(4):837-44. doi: 10.3892/ijo_00000397. Int J Oncol. 2009. PMID: 19724920 Free PMC article.
-
Reactive Oxygen Species and Targeted Therapy for Pancreatic Cancer.Oxid Med Cell Longev. 2016;2016:1616781. doi: 10.1155/2016/1616781. Epub 2016 Jan 3. Oxid Med Cell Longev. 2016. PMID: 26881012 Free PMC article. Review.
-
Metformin combined with aspirin significantly inhibit pancreatic cancer cell growth in vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and Bcl-2.Oncotarget. 2015 Aug 28;6(25):21208-24. doi: 10.18632/oncotarget.4126. Oncotarget. 2015. PMID: 26056043 Free PMC article.
-
Nitric Oxide-Releasing Aspirin Suppresses NF-κB Signaling in Estrogen Receptor Negative Breast Cancer Cells in Vitro and in Vivo.Molecules. 2015 Jul 9;20(7):12481-99. doi: 10.3390/molecules200712481. Molecules. 2015. PMID: 26184135 Free PMC article.
References
-
- Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of pancreas: extended lymphadenectomy or modified en bloc resection? ANZ J Surg. 2008;78:228–36. - PubMed
-
- Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–82. - PubMed
-
- Boeck S, Heinemann V. The role of second-line chemotherapy after gemcitabine failure in patients with advanced pancreatic cancer. Future Oncol. 2008;4:41–50. - PubMed
-
- Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, Kashfi K, Rigas B. Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res. 2006;66:4503–11. - PubMed
-
- Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol. 2007;23:55–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

