Characterizing aqueous solution conformations of a peptide backbone using Raman optical activity computations
- PMID: 18805935
- PMCID: PMC2599840
- DOI: 10.1529/biophysj.108.137596
Characterizing aqueous solution conformations of a peptide backbone using Raman optical activity computations
Abstract
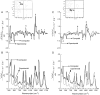
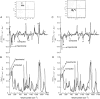
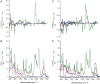
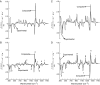
Mounting spectroscopic evidence indicates that alanine predominantly adopts extended polyproline II (PPII) conformations in short polypeptides. Here we analyze Raman optical activity (ROA) spectra of N-acetylalanine-N'-methylamide (Ala dipeptide) in H2O and D2O using density functional theory on Monte Carlo (MC) sampled geometries to examine the propensity of Ala dipeptide to adopt compact right-handed (alpha(R)) and left-handed (alpha(L)) helical conformations. The computed ROA spectra based on MC-sampled alpha(R) and PPII peptide conformations contain all the key spectral features found in the measured spectra. However, there is no significant similarity between the measured and computed ROA spectra based on the alpha(L)- and beta-conformations sampled by the MC methods. This analysis suggests that Ala dipeptide populates the alpha(R) and PPII conformations but no substantial population of alpha(L)- or beta-structures, despite sampling alpha(L)- and beta-structures in our MC simulations. Thus, ROA spectra combined with the theoretical analysis allow us to determine the dominant populated structures. Including explicit solute-solvent interactions in the theoretical analysis is essential for the success of this approach.
Figures


Similar articles
-
Raman optical activity of a cyclic dipeptide analyzed by quantum chemical calculations combined with molecular dynamics simulations.J Phys Chem B. 2014 Jun 19;118(24):6767-74. doi: 10.1021/jp503874z. Epub 2014 Jun 6. J Phys Chem B. 2014. PMID: 24873951
-
Role of solvent in determining conformational preferences of alanine dipeptide in water.J Am Chem Soc. 2004 Mar 3;126(8):2574-81. doi: 10.1021/ja039051x. J Am Chem Soc. 2004. PMID: 14982467
-
Conformational properties of the Pro-Gly motif in the D-Ala-l-Pro-Gly-D-Ala model peptide explored by a statistical analysis of the NMR, Raman, and Raman optical activity spectra.J Org Chem. 2008 Feb 15;73(4):1481-9. doi: 10.1021/jo702297y. Epub 2008 Jan 19. J Org Chem. 2008. PMID: 18205382
-
Polyproline II structure in proteins: identification by chiroptical spectroscopies, stability, and functions.Chirality. 2002 Nov;14(10):782-92. doi: 10.1002/chir.10153. Chirality. 2002. PMID: 12395395 Review.
-
Potential of Raman spectroscopic techniques to study proteins.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Sep 5;258:119712. doi: 10.1016/j.saa.2021.119712. Epub 2021 Apr 20. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33965670 Review.
Cited by
-
A statistical analysis of the PPII propensity of amino acid guests in proline-rich peptides.Biophys J. 2011 Feb 16;100(4):1083-93. doi: 10.1016/j.bpj.2010.12.3742. Biophys J. 2011. PMID: 21320454 Free PMC article.
-
The Polarizable Atomic Multipole-based AMOEBA Force Field for Proteins.J Chem Theory Comput. 2013;9(9):4046-4063. doi: 10.1021/ct4003702. J Chem Theory Comput. 2013. PMID: 24163642 Free PMC article.
-
Optimized molecular dynamics force fields applied to the helix-coil transition of polypeptides.J Phys Chem B. 2009 Jul 2;113(26):9004-15. doi: 10.1021/jp901540t. J Phys Chem B. 2009. PMID: 19514729 Free PMC article.
-
A coupled two-dimensional main chain torsional potential for protein dynamics: generation and implementation.J Mol Model. 2013 Sep;19(9):3647-57. doi: 10.1007/s00894-013-1879-8. Epub 2013 Jun 14. J Mol Model. 2013. PMID: 23765039
-
Optical signatures of molecular dissymmetry: combining theory with experiments to address stereochemical puzzles.Acc Chem Res. 2009 Jun 16;42(6):809-19. doi: 10.1021/ar8002859. Acc Chem Res. 2009. PMID: 19378940 Free PMC article.
References
-
- Chen, K., Z. Liu, C. Zhou, W. C. Bracken, and N. R. Kallenbach. 2007. Spin relaxation enhancement confirms dominance of extended conformations in short alanine peptides. Angew. Chem. Int. Ed. 46:9036–9039. - PubMed
-
- Shi, Z. S., K. Chen, Z. G. Liu, and N. R. Kallenbach. 2006. Conformation of the backbone in unfolded proteins. Chem. Rev. 106:1877–1897. - PubMed
-
- Makowska, J., S. Rodziewicz-Motowidlo, K. Baginska, J. A. Vila, A. Liwo, L. Chmurzynski, and H. A. Scheraga. 2006. Polyproline II conformation is one of many local conformational states and is not an overall conformation of unfolded peptides and proteins. Proc. Natl. Acad. Sci. USA. 103:1744–1749. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

