Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue
- PMID: 18805960
- PMCID: PMC2604790
- DOI: 10.1152/ajplung.90202.2008
Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue
Abstract
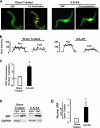
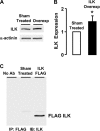
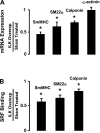
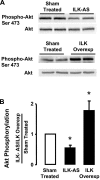
Phenotypic changes in airway smooth muscle occur with airway inflammation and asthma. These changes may be induced by alterations in the extracellular matrix that initiate signaling pathways mediated by integrin receptors. We hypothesized that integrin-linked kinase (ILK), a multidomain protein kinase that binds to the cytoplasmic tail of beta-integrins, may be an important mediator of signaling pathways that regulate the growth and differentiation state of airway smooth muscle. We disrupted signaling pathways mediated by ILK in intact differentiated tracheal muscle tissues by depleting ILK protein using ILK antisense. The depletion of ILK protein increased the expression of the smooth muscle differentiation marker genes myosin heavy chain (SmMHC), SM22alpha, and calponin and increased the expression of SmMHC protein. Conversely, the overexpression of ILK protein reduced the mRNA levels of SmMHC, SM22alpha, and calponin and SmMHC protein. Analysis by chromatin immunoprecipitation showed that the binding of the transcriptional regulator serum response factor (SRF) to the promoters of SmMHC, SM22alpha, and calponin genes was increased in ILK-depleted tissues and decreased in tissues overexpressing ILK. ILK depletion also increased the amount of SRF that localized within the nucleus. ILK depletion and overexpression, respectively, decreased and increased the activation of its downstream substrate protein kinase B (PKB/Akt). The pharmacological inhibition of Akt activity also increased SRF binding to the promoters of smooth muscle-specific genes and increased expression of smooth muscle proteins, suggesting that ILK may exert its effects by regulating the activity of Akt. We conclude that ILK is a critical regulator of airway smooth muscle differentiation. ILK may mediate signals from integrin receptors that control airway smooth muscle differentiation in response to alterations in the extracellular matrix.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

