Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque
- PMID: 18805978
- PMCID: PMC2593232
- DOI: 10.1128/JB.00983-08
Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque
Abstract
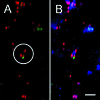
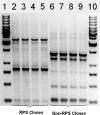
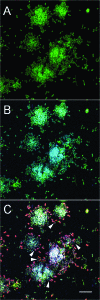
Streptococci and veillonellae occur in mixed-species colonies during formation of early dental plaque. One factor hypothesized to be important in assembly of these initial communities is coaggregation (cell-cell recognition by genetically distinct bacteria). Intrageneric coaggregation of streptococci occurs when a lectin-like adhesin on one streptococcal species recognizes a receptor polysaccharide (RPS) on the partner species. Veillonellae also coaggregate with streptococci. These genera interact metabolically; lactic acid produced by streptococci is a carbon source for veillonellae. To transpose these interactions from undisturbed dental plaque to an experimentally tractable in vitro biofilm model, a community consisting of RPS-bearing streptococci juxtaposed with veillonellae was targeted by quantum dot-based immunofluorescence and then micromanipulated off the enamel surface and cultured. Besides the expected antibody-reactive cell types, a non-antibody-reactive streptococcus invisible during micromanipulation was obtained. The streptococci were identified as Streptococcus oralis (RPS bearing) and Streptococcus gordonii (adhesin bearing). The veillonellae could not be cultivated; however, a veillonella 16S rRNA gene sequence was amplified from the original isolation mixture, and this sequence was identical to the sequence of the previously studied organism Veillonella sp. strain PK1910, an oral isolate in our culture collection. S. oralis coaggregated with S. gordonii by an RPS-dependent mechanism, and both streptococci coaggregated with PK1910, which was used as a surrogate during in vitro community reconstruction. The streptococci and strain PK1910 formed interdigitated three-species clusters when grown as a biofilm using saliva as the nutritional source. PK1910 grew only when streptococci were present. This study confirms that RPS-mediated intrageneric coaggregation occurs in the earliest stages of plaque formation by bringing bacteria together to create a functional community.
Figures



Comment in
-
Tracking dynamic interactions during plaque formation.J Bacteriol. 2008 Dec;190(24):7869-70. doi: 10.1128/JB.01344-08. Epub 2008 Oct 10. J Bacteriol. 2008. PMID: 18849426 Free PMC article. No abstract available.
References
-
- Bruchez, M., Jr., M. Moronne, P. Gin, S. Weiss, and A. P. Alivisatos. 1998. Semiconductor nanocrystals as fluorescent biological labels. Science 2812013-2016. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

