Mono-galloyl glucose derivatives are potent poly(ADP-ribose) glycohydrolase (PARG) inhibitors and partially reduce PARP-1-dependent cell death
- PMID: 18806807
- PMCID: PMC2607208
- DOI: 10.1038/bjp.2008.370
Mono-galloyl glucose derivatives are potent poly(ADP-ribose) glycohydrolase (PARG) inhibitors and partially reduce PARP-1-dependent cell death
Abstract
Background and purpose: Maintenance of poly(ADP-ribose) (PAR) polymers at homoeostatic levels by PAR glycohydrolase (PARG) is central in cell functioning and survival. Yet the pharmacological relevance of PARG inhibitors is still debated. Gallotannin, a complex mixture of hydrolysable tannins from oak gall, inhibits PARG but which of its constituents is responsible for the inhibition and whether the pharmacodynamic properties are due to its antioxidant properties, has not yet been established.
Experimental approach: A structure-activity relationship study was conducted on different natural and synthetic tannins/galloyl derivatives as potential PARG inhibitors, using a novel in vitro enzymic assay. Cytotoxicity was assayed in cultured HeLa cells.
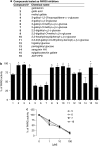
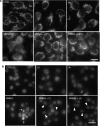
Key results: Mono-galloyl glucose compounds were potent inhibitors of PARG, with activities similar to that of ADP-(hydroxymethyl) pyrrolidinediol, the most potent PARG inhibitor yet identified. When tested on HeLa cells exposed to the PAR polymerase (PARP)-1-activating compound 1-methyl-3-nitro-1-nitrosoguanidine (MNNG), 3-galloyl glucose weakly inhibited PAR degradation. Conversely, the more lipophilic, 3-galloyl-1,2-O-isopropylidene glucose, despite being inactive on the pure enzyme, efficiently prolonged the half-life of the polymers in intact HeLa cells. Also, PARG inhibitors, but not radical scavengers, reduced, in part, cell death caused by MNNG.
Conclusions and implications: Taken together, our findings identify mono-galloyl glucose derivatives as potent PARG inhibitors, and emphasize the active function of this enzyme in cell death.
Figures





Similar articles
-
Poly(ADP-ribose) glycohydrolase as a target for neuroprotective intervention: assessment of currently available pharmacological tools.Eur J Pharmacol. 2004 Aug 16;497(1):7-16. doi: 10.1016/j.ejphar.2004.06.042. Eur J Pharmacol. 2004. PMID: 15321729
-
Tannins elevate the level of poly(ADP-ribose) in HeLa cell extracts.Arch Biochem Biophys. 2004 May 1;425(1):115-21. doi: 10.1016/j.abb.2004.02.024. Arch Biochem Biophys. 2004. PMID: 15081900
-
Inhibition of poly(ADP-ribose) polymerase-1 or poly(ADP‑ribose) glycohydrolase individually, but not in combination, leads to improved chemotherapeutic efficacy in HeLa cells.Int J Oncol. 2013 Feb;42(2):749-56. doi: 10.3892/ijo.2012.1740. Epub 2012 Dec 17. Int J Oncol. 2013. PMID: 23254695 Free PMC article.
-
New Insights into the Roles of NAD+-Poly(ADP-ribose) Metabolism and Poly(ADP-ribose) Glycohydrolase.Curr Protein Pept Sci. 2016;17(7):668-682. doi: 10.2174/1389203717666160419150014. Curr Protein Pept Sci. 2016. PMID: 27817743 Review.
-
Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP) - Function in Genome Maintenance and Relevance of Inhibitors for Anti-cancer Therapy.Front Mol Biosci. 2020 Aug 28;7:191. doi: 10.3389/fmolb.2020.00191. eCollection 2020. Front Mol Biosci. 2020. PMID: 33005627 Free PMC article. Review.
Cited by
-
Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria.PLoS One. 2013 Oct 14;8(10):e76938. doi: 10.1371/journal.pone.0076938. eCollection 2013. PLoS One. 2013. PMID: 24155910 Free PMC article.
-
Selective down-regulation of nuclear poly(ADP-ribose) glycohydrolase.PLoS One. 2009;4(3):e4896. doi: 10.1371/journal.pone.0004896. Epub 2009 Mar 25. PLoS One. 2009. PMID: 19319190 Free PMC article.
-
A specific isoform of poly(ADP-ribose) glycohydrolase is targeted to the mitochondrial matrix by a N-terminal mitochondrial targeting sequence.Exp Cell Res. 2009 Dec 10;315(20):3477-85. doi: 10.1016/j.yexcr.2009.04.005. Epub 2009 Apr 21. Exp Cell Res. 2009. PMID: 19389396 Free PMC article.
-
ADP-ribose hydrolases: biological functions and potential therapeutic targets.Expert Rev Mol Med. 2024 Oct 8;26:e21. doi: 10.1017/erm.2024.17. Expert Rev Mol Med. 2024. PMID: 39375922 Free PMC article. Review.
-
Poly(ADP-ribose)glycohydrolase is an upstream regulator of Ca2+ fluxes in oxidative cell death.Cell Mol Life Sci. 2011 Apr;68(8):1455-66. doi: 10.1007/s00018-010-0533-1. Epub 2010 Sep 29. Cell Mol Life Sci. 2011. PMID: 20878536 Free PMC article.
References
-
- Affar EB, Germain M, Winstall E, Vodenicharov M, Shah RG, Salvesen GS, et al. Caspase-3-mediated processing of poly(ADP-ribose) glycohydrolase during apoptosis. J Biol Chem. 2001;276:2935–2942. - PubMed
-
- Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, et al. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–85. - PubMed
-
- Alvarez-Gonzalez R, Althaus FR. Poly(ADP-ribose) catabolism in mammalian cells exposed to DNA-damaging agents. Mutat Res. 1989;218:67–74. - PubMed
-
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

