Low doses of alpha 2-adrenoceptor antagonists augment spinal morphine analgesia and inhibit development of acute and chronic tolerance
- PMID: 18806811
- PMCID: PMC2607212
- DOI: 10.1038/bjp.2008.353
Low doses of alpha 2-adrenoceptor antagonists augment spinal morphine analgesia and inhibit development of acute and chronic tolerance
Abstract
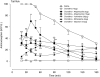
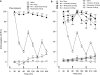
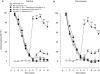
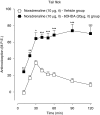
Background and purpose: Ultra-low doses of opioid receptor antagonists augment spinal morphine antinociception and block the induction of tolerance. Considering the evidence demonstrating functional and physical interactions between the opioid and alpha(2)-adrenoceptors, this study investigated whether ultra-low doses of alpha(2)-adrenoceptor antagonists also influence spinal morphine analgesia and tolerance.
Experimental approach: Effects of low doses of the competitive alpha(2)-adrenoceptor antagonists-atipamezole (0.08, 0.8 ng), yohimbine (0.02, 2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) were investigated on intrathecal morphine analgesia, as well as acute and chronic morphine antinociceptive tolerance using the rat tail flick and paw pressure tests.
Key results: At doses markedly lower than those producing alpha(2)-adrenoceptor blockade, atipamezole, yohimbine, mirtazapine and idazoxan, prolonged the antinociceptive effects of morphine. When co-administered with repeated acute spinal injections of morphine, all four agents blocked the induction of acute tolerance. Co-injection of atipamezole with morphine for 5 days inhibited the development of tolerance in a chronic treatment paradigm. Spinal administration of atipamezole also reversed established antinociceptive tolerance to morphine as indicated by the restoration of morphine antinociceptive potency. The effects of atipamezole on spinal morphine tolerance were not influenced by treatment with 6-hydroxydopamine.
Conclusions and implications: Low doses of competitive alpha(2)-adrenoceptor antagonists can augment acute morphine analgesia and block or reverse tolerance to spinal administration of morphine. These actions are interpreted in terms of their interaction with an opioid-alpha(2)-adrenoceptor complex, whose activity may have a function in the genesis of analgesic tolerance.
Figures




Similar articles
-
Intrathecal atipamezole augments the antinociceptive effect of morphine in rats.Anesth Analg. 2012 Jun;114(6):1353-8. doi: 10.1213/ANE.0b013e31824c727d. Epub 2012 May 3. Anesth Analg. 2012. PMID: 22556211
-
Analgesia, enhancement of spinal morphine antinociception, and inhibition of tolerance by ultra-low dose of the α2A-adrenoceptor selective antagonist BRL44408.Eur J Pharmacol. 2014 Nov 15;743:89-97. doi: 10.1016/j.ejphar.2014.08.040. Epub 2014 Sep 19. Eur J Pharmacol. 2014. PMID: 25242119
-
Stereo-selective inhibition of spinal morphine tolerance and hyperalgesia by an ultra-low dose of the alpha-2-adrenoceptor antagonist efaroxan.Eur J Pharmacol. 2013 Feb 28;702(1-3):227-34. doi: 10.1016/j.ejphar.2013.01.022. Epub 2013 Jan 29. Eur J Pharmacol. 2013. PMID: 23376415
-
Attenuation of tolerance to opioid-induced antinociception and protection against morphine-induced decrease of neurofilament proteins by idazoxan and other I2-imidazoline ligands.Br J Pharmacol. 1998 Sep;125(1):175-85. doi: 10.1038/sj.bjp.0702031. Br J Pharmacol. 1998. PMID: 9776358 Free PMC article.
-
The therapeutic potential of ultra-short-acting β-receptor antagonists in perioperative analgesic: Evidence from preclinical and clinical studies.Front Pharmacol. 2022 Oct 11;13:914710. doi: 10.3389/fphar.2022.914710. eCollection 2022. Front Pharmacol. 2022. PMID: 36304145 Free PMC article. Review.
Cited by
-
Mirtazapine Improves Locomotor Activity and Attenuates Neuropathic Pain Following Spinal Cord Injury in Rats via Neuroinflammation Modulation.Neurochem Res. 2024 Dec;49(12):3326-3341. doi: 10.1007/s11064-024-04240-7. Epub 2024 Sep 13. Neurochem Res. 2024. PMID: 39271550
-
Co-Evolution of Opioid and Adrenergic Ligands and Receptors: Shared, Complementary Modules Explain Evolution of Functional Interactions and Suggest Novel Engineering Possibilities.Life (Basel). 2021 Nov 10;11(11):1217. doi: 10.3390/life11111217. Life (Basel). 2021. PMID: 34833093 Free PMC article.
-
Mutual Enhancement of Opioid and Adrenergic Receptors by Combinations of Opioids and Adrenergic Ligands Is Reflected in Molecular Complementarity of Ligands: Drug Development Possibilities.Int J Mol Sci. 2019 Aug 24;20(17):4137. doi: 10.3390/ijms20174137. Int J Mol Sci. 2019. PMID: 31450631 Free PMC article.
-
Liquorice for pain?Ther Adv Psychopharmacol. 2021 Jul 16;11:20451253211024873. doi: 10.1177/20451253211024873. eCollection 2021. Ther Adv Psychopharmacol. 2021. PMID: 34349979 Free PMC article. Review.
-
Adrenergic Agonists Bind to Adrenergic-Receptor-Like Regions of the Mu Opioid Receptor, Enhancing Morphine and Methionine-Enkephalin Binding: A New Approach to "Biased Opioids"?Int J Mol Sci. 2018 Jan 17;19(1):272. doi: 10.3390/ijms19010272. Int J Mol Sci. 2018. PMID: 29342106 Free PMC article.
References
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

