Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus
- PMID: 18806877
- PMCID: PMC2533118
- DOI: 10.1371/journal.pone.0003256
Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus
Abstract
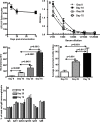
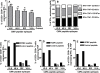
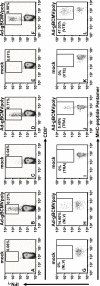
Based on the life-time cost to the health care system, the Institute of Medicine has assigned the highest priority for a vaccine to control human cytomegalovirus (HCMV) disease in transplant patients and new born babies. In spite of numerous attempts successful licensure of a HCMV vaccine formulation remains elusive. Here we have developed a novel chimeric vaccine strategy based on a replication-deficient adenovirus which encodes the extracellular domain of gB protein and multiple HLA class I & II-restricted CTL epitopes from HCMV as a contiguous polypeptide. Immunisation with this chimeric vaccine consistently generated strong HCMV-specific CD8(+) and CD4(+) T-cells which co-expressed IFN-gamma and TNF-alpha, while the humoral response induced by this vaccine showed strong virus neutralizing capacity. More importantly, immunization with adenoviral chimeric vaccine also afforded protection against challenge with recombinant vaccinia virus encoding HCMV antigens and this protection was associated with the induction of a pluripotent antigen-specific cellular and antibody response. Furthermore, in vitro stimulation with this adenoviral chimeric vaccine rapidly expanded multiple antigen-specific human CD8(+) and CD4(+) T-cells from healthy virus carriers. These studies demonstrate that the adenovirus chimeric HCMV vaccine provides an excellent platform for reconstituting protective immunity to prevent HCMV diseases in different clinical settings.
Conflict of interest statement
Figures





Similar articles
-
Ad-gBCMVpoly: A novel chimeric vaccine strategy for human cytomegalovirus-associated diseases.J Clin Virol. 2009 Dec;46 Suppl 4:S68-72. doi: 10.1016/j.jcv.2009.07.003. Epub 2009 Jul 30. J Clin Virol. 2009. PMID: 19646921
-
Attenuated poxvirus expressing three immunodominant CMV antigens as a vaccine strategy for CMV infection.J Clin Virol. 2006 Mar;35(3):324-31. doi: 10.1016/j.jcv.2005.09.018. Epub 2006 Jan 4. J Clin Virol. 2006. PMID: 16388983
-
Delineating the role of CD4+ T cells in the activation of human cytomegalovirus-specific immune responses following immunization with Ad-gBCMVpoly vaccine: implications for vaccination of immunocompromised individuals.J Gen Virol. 2010 Dec;91(Pt 12):2994-3001. doi: 10.1099/vir.0.025742-0. Epub 2010 Sep 1. J Gen Virol. 2010. PMID: 20810748
-
Human cytomegalovirus vaccine: time to look for alternative options.Trends Mol Med. 2006 Jan;12(1):26-33. doi: 10.1016/j.molmed.2005.11.006. Epub 2005 Dec 7. Trends Mol Med. 2006. PMID: 16337831 Review.
-
The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB.Vaccine. 2012 Nov 19;30(49):6980-90. doi: 10.1016/j.vaccine.2012.09.056. Epub 2012 Oct 3. Vaccine. 2012. PMID: 23041121 Review.
Cited by
-
Immunobiology of human cytomegalovirus: from bench to bedside.Clin Microbiol Rev. 2009 Jan;22(1):76-98, Table of Contents. doi: 10.1128/CMR.00034-08. Clin Microbiol Rev. 2009. PMID: 19136435 Free PMC article. Review.
-
Developing a Vaccine against Congenital Cytomegalovirus (CMV) Infection: What Have We Learned from Animal Models? Where Should We Go Next?Future Virol. 2013 Dec;8(12):1161-1182. doi: 10.2217/fvl.13.106. Future Virol. 2013. PMID: 24523827 Free PMC article.
-
Cytomegalovirus vaccines: at last, a major step forward.Herpes. 2009 Jan;15(3):44-5. Herpes. 2009. PMID: 19306601 Free PMC article.
-
Cellular immune therapy for viral infections in transplant patients.Indian J Med Res. 2013 Nov;138(5):796-807. Indian J Med Res. 2013. PMID: 24434332 Free PMC article. Review.
-
Discordance Between the Predicted Versus the Actually Recognized CD8+ T Cell Epitopes of HCMV pp65 Antigen and Aleatory Epitope Dominance.Front Immunol. 2021 Feb 9;11:618428. doi: 10.3389/fimmu.2020.618428. eCollection 2020. Front Immunol. 2021. PMID: 33633736 Free PMC article.
References
-
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. - PubMed
-
- Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet Gynecol Surv. 2002;57:245–256. - PubMed
-
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. - PubMed
-
- Stratton KR, Durch JS, Lawrence RS Committee to Study Priorities for Vaccine D. Vaccines for the 21st Century: A tool for decision making. Bethesda: National Academy Press; 2001. p. 476.
-
- Reddehase MJ. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat Rev Immunol. 2002 Nov;2(11):831–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

