Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole
- PMID: 18808119
- PMCID: PMC2700827
- DOI: 10.1021/ja803928m
Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole
Abstract
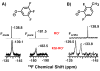
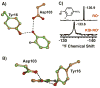
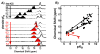
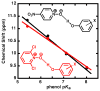
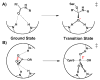
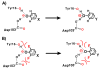
Enzymes are classically proposed to accelerate reactions by binding substrates within active-site environments that are structurally preorganized to optimize binding interactions with reaction transition states rather than ground states. This is a remarkably formidable task considering the limited 0.1-1 A scale of most substrate rearrangements. The flexibility of active-site functional groups along the coordinate of substrate rearrangement, the distance scale on which enzymes can distinguish structural rearrangement, and the energetic significance of discrimination on that scale remain open questions that are fundamental to a basic physical understanding of enzyme active sites and catalysis. We bring together 1.2-1.5 A resolution X-ray crystallography, (1)H and (19)F NMR spectroscopy, quantum mechanical calculations, and transition-state analogue binding measurements to test the distance scale on which noncovalent forces can constrain the structural relaxation or translation of side chains and ligands along a specific coordinate and the energetic consequences of such geometric constraints within the active site of bacterial ketosteroid isomerase (KSI). Our results strongly suggest that packing and binding interactions within the KSI active site can constrain local side-chain reorientation and prevent hydrogen bond shortening by 0.1 A or less. Further, this constraint has substantial energetic effects on ligand binding and stabilization of negative charge within the oxyanion hole. These results provide evidence that subtle geometric effects, indistinguishable in most X-ray crystallographic structures, can have significant energetic consequences and highlight the importance of using synergistic experimental approaches to dissect enzyme function.
Figures
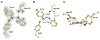


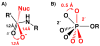

References
-
- Bolon DN, Voigt CA, Mayo SL. Curr Opin Chem Biol. 2002;6:125–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

