A role of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in cancer cells: a possible role to suppress cell proliferation
- PMID: 18808525
- PMCID: PMC2613979
- DOI: 10.1111/j.1365-2613.2008.00599.x
A role of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in cancer cells: a possible role to suppress cell proliferation
Abstract
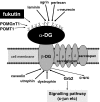
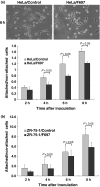
Fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy (FCMD), is presumably related to the glycosylation of alpha-dystroglycan (alpha-DG), involved in basement membrane formation. Hypoglycosylation of alpha-DG plays a key role for the pathogenesis of FCMD. On the other hand, fukutin and alpha-DG are also expressed in various non-neuromuscular tissues. Recently, a role of alpha-DG as a cancer suppressor has been proposed, because of a decrease of glycosylated alpha-DG in cancers. In this study, function of fukutin was investigated in two cancer cell lines, focusing on whether fukutin is involved in the glycosylation of alpha-DG in cancer cells and has any possible roles related to a cancer suppressor. Localization of fukutin and a result of laminin-binding assay after RNA interference suggest that fukutin may be involved in the glycosylation of alpha-DG in a small portion in these cancer cell lines. In Western blotting and immuno-electron microscopy, localization of fukutin in the nucleus was suggested in addition to the Golgi apparatus and/or endoplasmic reticulum. Immunohistochemically, there were more Ki-67-positive cells and more nuclear staining of phosphorylated c-jun after knockdown of fukutin in two cell lines. Fukutin appears to suppress cell proliferation through a system involving c-jun, although it is unclear this process is related to alpha-DG or not at present. The result may propose a possibility of another function of fukutin in addition to the glycosylation of alpha-DG in cancer cells.
Figures





Similar articles
-
Fukutin expression in mouse non-muscle somatic organs: its relationship to the hypoglycosylation of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy.Brain Dev. 2004 Oct;26(7):469-79. doi: 10.1016/j.braindev.2004.01.004. Brain Dev. 2004. PMID: 15351084
-
Co-localization of fukutin and alpha-dystroglycan in the mouse central nervous system.Brain Res Dev Brain Res. 2004 Sep 17;152(2):121-7. doi: 10.1016/j.devbrainres.2004.06.006. Brain Res Dev Brain Res. 2004. PMID: 15351499
-
Altered glycosylation of alpha-dystroglycan in neurons of Fukuyama congenital muscular dystrophy brains.Brain Res. 2006 Feb 23;1075(1):223-8. doi: 10.1016/j.brainres.2005.12.108. Epub 2006 Feb 7. Brain Res. 2006. PMID: 16466646
-
Functions of fukutin, a gene responsible for Fukuyama type congenital muscular dystrophy, in neuromuscular system and other somatic organs.Cent Nerv Syst Agents Med Chem. 2010 Jun 1;10(2):169-79. doi: 10.2174/187152410791196369. Cent Nerv Syst Agents Med Chem. 2010. PMID: 20518731 Review.
-
Expression and localization of fukutin, POMGnT1, and POMT1 in the central nervous system: consideration for functions of fukutin.Med Electron Microsc. 2004 Dec;37(4):200-7. doi: 10.1007/s00795-004-0260-5. Med Electron Microsc. 2004. PMID: 15614444 Review.
Cited by
-
Fukutin Protein Participates in Cell Proliferation by Enhancing Cyclin D1 Expression through Binding to the Transcription Factor Activator Protein-1: An In Vitro Study.Int J Mol Sci. 2021 Nov 10;22(22):12153. doi: 10.3390/ijms222212153. Int J Mol Sci. 2021. PMID: 34830034 Free PMC article.
-
Fukutin, identified by the Escherichia coli ampicillin secretion trap (CAST) method, participates in tumor progression in gastric cancer.Gastric Cancer. 2016 Apr;19(2):443-452. doi: 10.1007/s10120-015-0511-2. Epub 2015 Jul 30. Gastric Cancer. 2016. PMID: 26223471
-
Post-transcriptional regulation of fukutin in an astrocytoma cell line.Int J Exp Pathol. 2012 Feb;93(1):46-55. doi: 10.1111/j.1365-2613.2011.00799.x. Int J Exp Pathol. 2012. PMID: 22264285 Free PMC article.
-
Expression in retinal neurons of fukutin and FKRP, the protein products of two dystroglycanopathy-causative genes.Mol Vis. 2018 Jan 20;24:43-58. eCollection 2018. Mol Vis. 2018. PMID: 29416295 Free PMC article.
-
Involvement of abnormal dystroglycan expression and matriglycan levels in cancer pathogenesis.Cancer Cell Int. 2022 Dec 9;22(1):395. doi: 10.1186/s12935-022-02812-7. Cancer Cell Int. 2022. PMID: 36494657 Free PMC article. Review.
References
-
- Akasaka-Manya K, Manya H, Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem. Biophys. Res. Commun. 2004;325:75–79. - PubMed
-
- Cattoretti G, Becker MHG, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J. Pathol. 1992;168:357–363. - PubMed
-
- Chiyonobu T, Sasaki J, Nagai Y, et al. Effects of fukutin deficiency in the developing brain. Neuromuscul. Disord. 2005;15:416–426. - PubMed
-
- El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

