Role of inflammation in right ventricular damage and repair following experimental pulmonary embolism in rats
- PMID: 18808531
- PMCID: PMC2613983
- DOI: 10.1111/j.1365-2613.2008.00610.x
Role of inflammation in right ventricular damage and repair following experimental pulmonary embolism in rats
Abstract
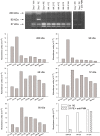
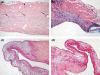
Right ventricular (RV) dysfunction is associated with poor clinical outcome following pulmonary embolism (PE). Previous studies in our laboratory show that influx of neutrophils contributes to acute RV damage seen in an 18 h rat model of PE. The present study describes the further progression of inflammation over 6 weeks and compares the neutrophil and monocyte responses. The RV outflow tract became white in colour by day 1 with influx of neutrophils (tissue myeloperoxidase activity increased 17-fold) and mononuclear cells with characteristics of M1 phenotype (high in Ccl20, Cxcl10, CcR2, MHCII, DNA microarray analysis). Matrix metalloproteinase activities were increased and tissue was thinned to produce a translucent appearance in weeks 1 through 6 in 40% of hearts. RV contractile function was significantly reduced at 6 weeks of PE. In this later phase, there was accumulation of myofibroblasts, the presence of mononuclear cells with M2 characteristics (high in scavenger mannose receptors, macrophage galactose lectin 1, PDGFR1, PDGFRbeta), enrichment of the subendocardial region of the RV outflow tract with neovesels (alpha-smooth muscle immunohistochemistry) and deposition of collagen fibres (picrosirius red staining) beginning scar formation. Thus, while neutrophil response is associated with the early, acute inflammatory events, macrophage cells continue to be present during the proliferative phase and initial deposition of collagen in this model, changing from the M1 to the M2 phenotype. This suggests that the macrophage cell response is biphasic.
Figures





Similar articles
-
Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats.J Mol Cell Cardiol. 2006 Aug;41(2):296-307. doi: 10.1016/j.yjmcc.2006.05.011. Epub 2006 Jun 30. J Mol Cell Cardiol. 2006. PMID: 16814320
-
Proinflammatory events in right ventricular damage during pulmonary embolism: effects of treatment with ketorolac in rats.J Cardiovasc Pharmacol. 2009 Sep;54(3):246-52. doi: 10.1097/FJC.0b013e3181b2b699. J Cardiovasc Pharmacol. 2009. PMID: 19620882
-
Inhibition of CINC-1 decreases right ventricular damage caused by experimental pulmonary embolism in rats.J Immunol. 2007 Dec 1;179(11):7820-6. doi: 10.4049/jimmunol.179.11.7820. J Immunol. 2007. PMID: 18025228
-
Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension.J Card Fail. 2010 Mar;16(3):250-9. doi: 10.1016/j.cardfail.2009.11.008. Epub 2010 Jan 15. J Card Fail. 2010. PMID: 20206901 Review.
-
Assessment of right ventricular systolic function by tissue Doppler echocardiography.Dan Med J. 2012 Mar;59(3):B4409. Dan Med J. 2012. PMID: 22381093 Review.
Cited by
-
Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement.Am J Respir Crit Care Med. 2018 Aug 15;198(4):e15-e43. doi: 10.1164/rccm.201806-1160ST. Am J Respir Crit Care Med. 2018. PMID: 30109950 Free PMC article.
-
Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure.Chest. 2009 Nov;136(5):1202-1210. doi: 10.1378/chest.08-2988. Epub 2009 Jun 19. Chest. 2009. PMID: 19542256 Free PMC article.
-
Association of Blood Leukocytes and Hemoglobin with Hospital Mortality in Acute Pulmonary Embolism.J Clin Med. 2023 Sep 28;12(19):6269. doi: 10.3390/jcm12196269. J Clin Med. 2023. PMID: 37834913 Free PMC article.
-
Regulation of the Immune Microenvironment by an NLRP3 Inhibitor Contributes to Attenuation of Acute Right Ventricular Failure in Rats with Pulmonary Arterial Hypertension.J Inflamm Res. 2021 Nov 2;14:5699-5711. doi: 10.2147/JIR.S336964. eCollection 2021. J Inflamm Res. 2021. PMID: 34754216 Free PMC article.
-
Utility of Blood Cellular Indices in the Risk Stratification of Patients Presenting with Acute Pulmonary Embolism.Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211052292. doi: 10.1177/10760296211052292. Clin Appl Thromb Hemost. 2021. PMID: 34846193 Free PMC article.
References
-
- Dack S, Master AM, Horn H, Grishman A, Field LE. Acute coronary insufficiency due to pulmonary embolism. Am. J. Med. 1949;7:464–477. - PubMed
-
- Dell'Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr. Probl. Cardiol. 1991;16:653–720. - PubMed
-
- Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid. Redox Signal. 2006;8:1907–1939. - PubMed
-
- Giannitsis E, Muller-Bardorff M, Kurowski V, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation. 2000;102:211–217. - PubMed
-
- Gold FL, Bache RJ. Transmural right ventricular blood flow during acute pulmonary artery hypertension in the sedated dog. Evidence for subendocardial ischemia despite residual vasodilator reserve. Circ. Res. 1982;51:196–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

