Generation of diverse neuronal subtypes in cloned populations of stem-like cells
- PMID: 18808670
- PMCID: PMC2556672
- DOI: 10.1186/1471-213X-8-89
Generation of diverse neuronal subtypes in cloned populations of stem-like cells
Abstract
Background: The central nervous tissue contains diverse subtypes of neurons with characteristic morphological and physiological features and different neurotransmitter phenotypes. The generation of neurons with defined neurotransmitter phenotypes seems to be governed by factors differently expressed along the anterior-posterior and dorsal-ventral body axes. The mechanisms of the cell-type determination, however, are poorly understood. Selected neuronal phenotypes had been generated from embryonic stem (ES) cells, but similar results were not obtained on more restricted neural stem cells, presumably due to the lack of homogeneous neural stem cell populations as a starting material.
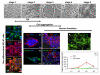
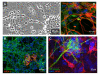
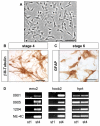
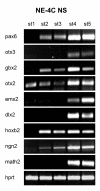
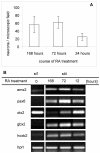
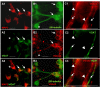
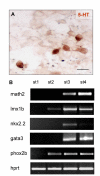
Results: In the presented work, the establishment of different neurotransmitter phenotypes was investigated in the course of in vitro induced neural differentiation of a one-cell derived neuroectodermal cell line, in conjunction with the activation of various region-specific genes. For comparison, similar studies were carried out on the R1 embryonic stem (ES) and P19 multipotent embryonic carcinoma (EC) cells. In response to a short treatment with all-trans retinoic acid, all cell lines gave rise to neurons and astrocytes. Non-induced neural stem cells and self-renewing cells persisting in differentiated cultures, expressed "stemness genes" along with early embryonic anterior-dorsal positional genes, but did not express the investigated CNS region-specific genes. In differentiating stem-like cell populations, on the other hand, different region-specific genes, those expressed in non-overlapping regions along the body axes were activated. The potential for diverse regional specifications was induced in parallel with the initiation of neural tissue-type differentiation. In accordance with the wide regional specification potential, neurons with different neurotransmitter phenotypes developed. Mechanisms inherent to one-cell derived neural stem cell populations were sufficient to establish glutamatergic and GABAergic neuronal phenotypes but failed to manifest cathecolaminergic neurons.
Conclusion: The data indicate that genes involved in positional determination are activated along with pro-neuronal genes in conditions excluding any outside influences. Interactions among progenies of one cell derived neural stem cells are sufficient for the activation of diverse region specific genes and initiate different routes of neuronal specification.
Figures




Similar articles
-
Expression profile of an operationally-defined neural stem cell clone.Exp Neurol. 2005 Aug;194(2):320-32. doi: 10.1016/j.expneurol.2005.04.018. Exp Neurol. 2005. PMID: 15992799
-
Human neurospheres derived from the fetal central nervous system are regionally and temporally specified but are not committed.Exp Neurol. 2006 May;199(1):222-35. doi: 10.1016/j.expneurol.2006.03.015. Epub 2006 May 22. Exp Neurol. 2006. PMID: 16714017
-
Neural cells derived by in vitro differentiation of P19 and embryonic stem cells.Perspect Dev Neurobiol. 1998;5(2-3):175-8. Perspect Dev Neurobiol. 1998. PMID: 9777634 Review.
-
Analysis of the expression and function of BRINP family genes during neuronal differentiation in mouse embryonic stem cell-derived neural stem cells.J Neurosci Res. 2010 May 15;88(7):1387-93. doi: 10.1002/jnr.22315. J Neurosci Res. 2010. PMID: 20025061
-
Stemness is only a state of the cell.Cold Spring Harb Symp Quant Biol. 2008;73:227-34. doi: 10.1101/sqb.2008.73.042. Epub 2009 Jan 15. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19150961 Review.
Cited by
-
Foxm1 transcription factor is required for maintenance of pluripotency of P19 embryonal carcinoma cells.Nucleic Acids Res. 2010 Dec;38(22):8027-38. doi: 10.1093/nar/gkq715. Epub 2010 Aug 11. Nucleic Acids Res. 2010. PMID: 20702419 Free PMC article.
-
Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex.Nat Protoc. 2011 Feb;6(2):214-28. doi: 10.1038/nprot.2010.188. Epub 2011 Feb 3. Nat Protoc. 2011. PMID: 21293461
-
MiR-302/367 regulate neural progenitor proliferation, differentiation timing, and survival in neurulation.Dev Biol. 2015 Dec 1;408(1):140-50. doi: 10.1016/j.ydbio.2015.09.020. Epub 2015 Oct 9. Dev Biol. 2015. PMID: 26441343 Free PMC article.
-
Gsx1 promotes locomotor functional recovery after spinal cord injury.Mol Ther. 2021 Aug 4;29(8):2469-2482. doi: 10.1016/j.ymthe.2021.04.027. Epub 2021 Apr 23. Mol Ther. 2021. PMID: 33895323 Free PMC article.
-
Retinoid machinery in distinct neural stem cell populations with different retinoid responsiveness.Stem Cells Dev. 2013 Oct 15;22(20):2777-93. doi: 10.1089/scd.2012.0422. Epub 2013 Jul 24. Stem Cells Dev. 2013. PMID: 23734950 Free PMC article.
References
-
- Shimamura K, Hartigan D, Martinez S, Puelles L, Rubenstein J. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

