Changes in GnRH I, bradykinin and their receptors and GnIH in the ovary of Calotes versicolor during reproductive cycle
- PMID: 18809405
- PMCID: PMC7927428
- DOI: 10.1016/j.ygcen.2008.08.016
Changes in GnRH I, bradykinin and their receptors and GnIH in the ovary of Calotes versicolor during reproductive cycle
Abstract
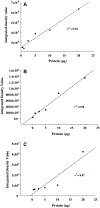
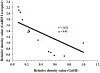
The aim of this study was to investigate changes in the abundance of gonadotrophin releasing hormone I (GnRH I) and GnRH I receptor in the ovary of Calotes versicolor during the reproductive cycle and correlate them with the changes in gonadotrophin inhibitory hormone (GnIH), bradykinin and bradykinin B(2) receptor in order to understand their interaction during ovarian cycle. GnRH I, bradykinin and their receptors and GnIH, were localized immunohistochemically in the ovary. Relative intensity of these peptides was estimated from the contralateral ovary using slot/Western blot followed by densitometry. The immunostaining of GnRH I, bradykinin and their receptors and GnIH were localized in the granulosa cells of previtellogenic follicles and stroma cells, whereas in the peripheral part of the cytoplasm in oocytes of vitellogenic and ovulatory follicles. The GnRH I immunostaining was relatively higher in inactive phase, but was low during active preovulatory phase suggesting inverse correlation with circulating estradiol level. The study showed a positive correlation between the expression pattern of GnRH I and GnIH, but showed a negative correlation between GnIH with GnRH I receptor in the ovary. This study further suggests a possibility for bradykinin regulating GnRH I synthesis in the ovary. An increase in the immunostaining of both GnRH I and GnIH in the oocyte prior to ovulation suggests their involvement in the oocyte maturation. It is thus concluded that the ovary of C. versicolor possesses GnRH I-GnIH-bradykinin system and interaction between these neuropeptides may be involved in the regulation of follicular development and oocyte maturation.
Figures



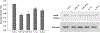
Similar articles
-
Cellular localization and seasonal variation of GnRH and Bradykinin in the ovary of Heteropneustes fossilis (Bloch.) during its reproductive cycle.Theriogenology. 2024 Jul 15;223:89-97. doi: 10.1016/j.theriogenology.2024.04.016. Epub 2024 Apr 25. Theriogenology. 2024. PMID: 38692038
-
Immunohistochemical localization of GnRH and RFamide-related peptide-3 in the ovaries of mice during the estrous cycle.J Mol Histol. 2011 Oct;42(5):371-81. doi: 10.1007/s10735-011-9340-8. Epub 2011 Jul 19. J Mol Histol. 2011. PMID: 21769536 Free PMC article.
-
Localization of gonadotrophin-releasing hormone I, bradykinin and their receptors in the ovaries of non-mammalian vertebrates.Reproduction. 2007 May;133(5):969-81. doi: 10.1530/REP-06-0106. Reproduction. 2007. PMID: 17616726
-
Gonadotropin-inhibitory hormone (GnIH) in the amphibian brain and its relationship with the gonadotropin releasing hormone (GnRH) system: An overview.Gen Comp Endocrinol. 2017 Jan 1;240:69-76. doi: 10.1016/j.ygcen.2016.09.006. Epub 2016 Sep 22. Gen Comp Endocrinol. 2017. PMID: 27667155 Review.
-
Extrapituitary effects of GnRH antagonists in assisted reproduction: a review.Reprod Biomed Online. 2005 Feb;10(2):230-4. doi: 10.1016/s1472-6483(10)60945-5. Reprod Biomed Online. 2005. PMID: 15823230 Review.
Cited by
-
RFRP3 influences basal lamina degradation, cellular death, and progesterone secretion in cultured preantral ovarian follicles from the domestic cat.PeerJ. 2019 Aug 23;7:e7540. doi: 10.7717/peerj.7540. eCollection 2019. PeerJ. 2019. PMID: 31497402 Free PMC article.
-
Expression and functional analysis of GnRH at the onset of puberty in sheep.Arch Anim Breed. 2022 Jul 20;65(3):249-257. doi: 10.5194/aab-65-249-2022. eCollection 2022. Arch Anim Breed. 2022. PMID: 36035881 Free PMC article.
-
The Role of RFamide-Related Peptide-3 in Age-Related Reproductive Decline in Female Rats.Front Endocrinol (Lausanne). 2016 Jun 21;7:71. doi: 10.3389/fendo.2016.00071. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27445974 Free PMC article.
-
GnIH Control of Feeding and Reproductive Behaviors.Front Endocrinol (Lausanne). 2016 Dec 27;7:170. doi: 10.3389/fendo.2016.00170. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 28082949 Free PMC article. Review.
-
A review of the evolution of viviparity in squamate reptiles: the past, present and future role of molecular biology and genomics.J Comp Physiol B. 2011 Jul;181(5):575-94. doi: 10.1007/s00360-011-0584-0. Epub 2011 May 15. J Comp Physiol B. 2011. PMID: 21573966 Review.
References
-
- Bauer-Dantoin AC, Jameson JL, 1995. Gonadotropin-releasing hormone receptor messenger ribonucleic acid expression in the ovary during the rat estrous cycle. Endocrinology 136, 4432–4438. - PubMed
-
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC, 2003. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. Journal of Neuroendocrinology 15, 794–802. - PubMed
-
- Bentley GE, Kriegsfeld LJ, Osugi T, Ukena K, O’Brien S, Perfito N, Moore IT, Tsutsui K, Wingfield JC, 2006. Interactions of gonadotropin-releasing hormone (GnRH I) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. Journal of Experimental Zoology A Comparative Experimental Biology 305, 807–814. - PubMed
-
- Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K, 2008. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. General and Comparative Endocrinology 156, 34–43. - PubMed
-
- Billig H, Furuta I, Hsueh AJ, 1994. Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulose cells. Endocrinology 134, 245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

