Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo
- PMID: 18809571
- PMCID: PMC2593383
- DOI: 10.1128/MCB.00732-08
Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo
Abstract
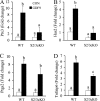
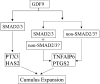
Transforming growth factor beta (TGF-beta) superfamily members are critical in maintaining cell growth and differentiation in the ovary. Although signaling of activins, TGF-betas, growth differentiation factor 9, and nodal converge preferentially to SMAD2 and SMAD3, the in vivo functions and redundancy of these SMADs in the ovary and female reproduction remain largely unidentified. To circumvent the deleterious phenotypic aspects of ubiquitous deletion of Smad2 and Smad3, a conditional knockout strategy was formulated to selectively inactivate Smad2, Smad3, or both Smad2 and Smad3 in ovarian granulosa cells. While granulosa cell ablation of individual Smad2 or Smad3 caused insignificant changes in female fertility, deletion of both Smad2 and Smad3 led to dramatically reduced female fertility and fecundity. These defects were associated with the disruption of multiple ovarian processes, including follicular development, ovulation, and cumulus cell expansion. Furthermore, the impaired expansion of cumulus cells may be partially associated with altered cumulus expansion-related transcripts that are regulated by SMAD2/3 signaling. Our results indicate that SMAD2 and SMAD3 function redundantly in vivo to maintain normal female fertility and further support the involvement of an intraovarian SMAD2/3 pathway in mediating oocyte-produced signals essential for coordinating key events of the ovulatory process.
Figures
Similar articles
-
Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development.J Immunol. 2010 Jul 15;185(2):842-55. doi: 10.4049/jimmunol.0904100. Epub 2010 Jun 14. J Immunol. 2010. PMID: 20548029
-
Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion.Biol Reprod. 2007 May;76(5):848-57. doi: 10.1095/biolreprod.106.057471. Epub 2006 Dec 27. Biol Reprod. 2007. PMID: 17192514
-
Smad2 and Smad3 are redundantly essential for the suppression of iNOS synthesis in macrophages by regulating IRF3 and STAT1 pathways.Int Immunol. 2012 Apr;24(4):253-65. doi: 10.1093/intimm/dxr126. Epub 2012 Feb 13. Int Immunol. 2012. PMID: 22331441
-
A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling.J Cell Biochem. 2007 May 1;101(1):9-33. doi: 10.1002/jcb.21255. J Cell Biochem. 2007. PMID: 17340614 Review.
-
From Follicular Development and Ovulation to Ovarian Cancers: An Unexpected Journey.Vitam Horm. 2018;107:453-472. doi: 10.1016/bs.vh.2018.01.019. Epub 2018 Feb 23. Vitam Horm. 2018. PMID: 29544640 Review.
Cited by
-
Transcriptome Dynamics and Cell Dialogs Between Oocytes and Granulosa Cells in Mouse Follicle Development.Genomics Proteomics Bioinformatics. 2024 Jul 2;22(2):qzad001. doi: 10.1093/gpbjnl/qzad001. Genomics Proteomics Bioinformatics. 2024. PMID: 38955498 Free PMC article.
-
Activin A promotes hyaluronan production and upregulates versican expression in human granulosa cells†.Biol Reprod. 2022 Aug 9;107(2):458-473. doi: 10.1093/biolre/ioac070. Biol Reprod. 2022. PMID: 35403677 Free PMC article.
-
Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation.Dev Cell. 2021 Aug 9;56(15):2237-2251.e6. doi: 10.1016/j.devcel.2021.06.021. Epub 2021 Jul 16. Dev Cell. 2021. PMID: 34273276 Free PMC article.
-
Evaluation of circulating microRNA profiles in Brazilian women with polycystic ovary syndrome: A preliminary study.PLoS One. 2022 Oct 7;17(10):e0275031. doi: 10.1371/journal.pone.0275031. eCollection 2022. PLoS One. 2022. PMID: 36206272 Free PMC article.
-
Potential Candidate Genes Associated with Litter Size in Goats: A Review.Animals (Basel). 2025 Jan 2;15(1):82. doi: 10.3390/ani15010082. Animals (Basel). 2025. PMID: 39795025 Free PMC article. Review.
References
-
- Andreu-Vieyra, C., R. Chen, and M. M. Matzuk. 2007. Effects of granulosa cell-specific deletion of Rb in Inha-alpha null female mice. Endocrinology. 1483837-3849. - PubMed
-
- Ashcroft, G. S., S. J. Mills, K. C. Flanders, L. A. Lyakh, M. A. Anzano, S. C. Gilliver, and A. B. Roberts. 2003. Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Repair Regen. 11468-473. - PubMed
-
- Billiar, R. B., J. B. St Clair, N. C. Zachos, M. G. Burch, E. D. Albrecht, and G. J. Pepe. 2004. Localization and developmental expression of the activin signal transduction proteins Smads 2, 3, and 4 in the baboon fetal ovary. Biol. Reprod. 70586-592. - PubMed
-
- Boerboom, D., M. Paquet, M. Hsieh, J. Liu, S. P. Jamin, R. R. Behringer, J. Sirois, M. M. Taketo, and J. S. Richards. 2005. Misregulated Wnt/β-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 659206-9215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
