MCAK-independent functions of ch-Tog/XMAP215 in microtubule plus-end dynamics
- PMID: 18809577
- PMCID: PMC2593372
- DOI: 10.1128/MCB.01040-08
MCAK-independent functions of ch-Tog/XMAP215 in microtubule plus-end dynamics
Abstract
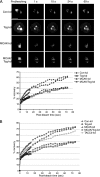
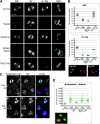
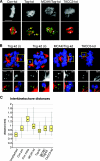
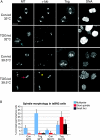
The formation of a functional bipolar mitotic spindle is essential for genetic integrity. In human cells, the microtubule polymerase XMAP215/ch-Tog ensures spindle bipolarity by counteracting the activity of the microtubule-depolymerizing kinesin XKCM1/MCAK. Their antagonistic effects on microtubule polymerization confer dynamic instability on microtubules assembled in cell-free systems. It is, however, unclear if a similar interplay governs microtubule behavior in mammalian cells in vivo. Using real-time analysis of spindle assembly, we found that ch-Tog is required to produce or maintain long centrosomal microtubules after nuclear-envelope breakdown. In the absence of ch-Tog, microtubule assembly at centrosomes was impaired and microtubules were nondynamic. Interkinetochore distances and the lengths of kinetochore fibers were also reduced in these cells. Codepleting MCAK with ch-Tog improved kinetochore fiber length and interkinetochore separation but, surprisingly, did not rescue centrosomal microtubule assembly and microtubule dynamics. Our data therefore suggest that ch-Tog has at least two distinct roles in spindle formation. First, it protects kinetochore microtubules from depolymerization by MCAK. Second, ch-Tog plays an essential role in centrosomal microtubule assembly, a function independent of MCAK activity. Thus, the notion that the antagonistic activities of MCAK and ch-Tog determine overall microtubule stability is too simplistic to apply to human cells.
Figures


References
-
- Al-Bassam, J., N. A. Larsen, A. A. Hyman, and S. C. Harrison. 2007. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15355-362. - PubMed
-
- Andrews, P. D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J. R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6253-268. - PubMed
-
- Barr, A. R., and F. Gergely. 2007. Aurora-A: the maker and breaker of spindle poles. J. Cell Sci. 1202987-2996. - PubMed
-
- Bastiaens, P., M. Caudron, P. Niethammer, and E. Karsenti. 2006. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 16125-134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
