Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens
- PMID: 18809613
- PMCID: PMC2652090
- DOI: 10.1200/JCO.2008.16.5449
Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens
Abstract
Purpose: The two approved treatments for patients with metastatic melanoma, interleukin (IL)-2 and dacarbazine, mediate objective response rates of 12% to 15%. We previously reported that adoptive cell therapy (ACT) with autologous antitumor lymphocytes in lymphodepleted hosts mediated objective responses in 51% of 35 patients. Here, we update that study and evaluate the safety and efficacy of two increased-intensity myeloablative lymphodepleting regimens.
Patients and methods: We performed two additional sequential trials of ACT with autologous tumor-infiltrating lymphocytes (TIL) in patients with metastatic melanoma. Increasing intensity of host preparative lymphodepletion consisting of cyclophosphamide and fludarabine with either 2 (25 patients) or 12 Gy (25 patients) of total-body irradiation (TBI) was administered before cell transfer. Objective response rates by Response Evaluation Criteria in Solid Tumors (RECIST) and survival were evaluated. Immunologic correlates of effective treatment were studied.
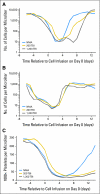
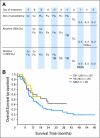
Results: Although nonmyeloablative chemotherapy alone showed an objective response rate of 49%, when 2 or 12 Gy of TBI was added, the response rates were 52% and 72% respectively. Responses were seen in all visceral sites including brain. There was one treatment-related death in the 93 patients. Host lymphodepletion was associated with increased serum levels of the lymphocyte homeostatic cytokines IL-7 and IL-15. Objective responses were correlated with the telomere length of the transferred cells.
Conclusion: Host lymphodepletion followed by autologous TIL transfer and IL-2 results in objective response rates of 50% to 70% in patients with metastatic melanoma refractory to standard therapies.
Figures

References
-
- Tsao H, Atkins MB, Sober AJ: Management of cutaneous melanoma. N Engl J Med 351:998-1012, 2004 - PubMed
-
- Zippelius A, Batard P, Rubio-Godoy V, et al: Effector function of human tumor-specific CD8 T cells in melanoma lesions: A state of local functional tolerance. Cancer Res 64:2865-2873, 2004 - PubMed
-
- Topalian SL, Muul LM, Solomon D, et al: Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods 102:127-141, 1987 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

