Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups
- PMID: 18809614
- PMCID: PMC2652093
- DOI: 10.1200/JCO.2008.16.4855
Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups
Abstract
Purpose: Adjuvant chemotherapy for resected non-small-cell lung cancer (NSCLC) is now accepted on the basis of several randomized clinical trials (RCTs) that demonstrated improved survival. Although there is strong evidence that adjuvant chemotherapy is effective in stages II and IIIA NSCLC, its utility in stage IB disease is unclear. This report provides a mature analysis of Cancer and Leukemia Group B (CALGB) 9633, the only RCT designed specifically for stage IB NSCLC.
Patients and methods: Within 4 to 8 weeks of resection, patients were randomly assigned to adjuvant chemotherapy or observation. Eligible patients had pathologically confirmed T2N0 NSCLC and had undergone lobectomy or pneumonectomy. Chemotherapy consisted of paclitaxel 200 mg/m(2) intravenously over 3 hours and carboplatin at an area under the curve dose of 6 mg/mL per minute intravenously over 45 to 60 minutes every 3 weeks for four cycles. The primary end point was overall survival.
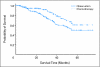
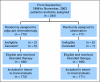
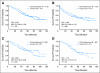
Results: Three hundred-forty-four patients were randomly assigned. Median follow-up was 74 months. Groups were well-balanced with regard to demographics, histology, and extent of surgery. Grades 3 to 4 neutropenia were the predominant toxicity; there were no treatment-related deaths. Survival was not significantly different (hazard ratio [HR], 0.83; CI, 0.64 to 1.08; P = .12). However, exploratory analysis demonstrated a significant survival difference in favor of adjuvant chemotherapy for patients who had tumors > or = 4 cm in diameter (HR, 0.69; CI, 0.48 to 0.99; P = .043).
Conclusion: Because a significant survival advantage was not observed across the entire cohort, adjuvant chemotherapy should not be considered standard care in stage IB NSCLC. Given the magnitude of observed survival differences, CALGB 9633 was underpowered to detect small but clinically meaningful improvements. A statistically significant survival advantage for patients who had tumors > or = 4 cm supports consideration of adjuvant paclitaxel/carboplatin for stage IB patients who have large tumors.
Figures
Comment in
-
Adjuvant chemotherapy for non-small-cell lung cancer: a fading effect?J Clin Oncol. 2008 Nov 1;26(31):5014-7. doi: 10.1200/JCO.2008.18.1081. Epub 2008 Sep 22. J Clin Oncol. 2008. PMID: 18809602 No abstract available.
-
Current issues in adjuvant chemotherapy for resected, stage IB non-small-cell lung cancer.Future Oncol. 2009 Feb;5(1):19-22. doi: 10.2217/14796694.5.1.19. Future Oncol. 2009. PMID: 19243293 No abstract available.
-
CALGB 9633: an underpowered trial with a methodologically questionable conclusion.J Clin Oncol. 2009 May 1;27(13):2300-1; author reply 2301-2. doi: 10.1200/JCO.2008.21.1565. Epub 2009 Mar 30. J Clin Oncol. 2009. PMID: 19332712 No abstract available.
References
-
- Le Chevalier T, et al: Results of the Randomized International Adjuvant Lung Cancer Trial (IALT): cisplatin-based chemotherapy (CT) versus no CT in 1867 patients with resected non–small-cell lung cancer. Proc Am Soc Clin Oncol 22:2, 2003. (abstr 6)
-
- Arriagada R, Bergman B, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer: The International Adjuvant Lung Cancer Trial (IALT) Collaborative Group. N Engl J Med 350:351-360, 2004 - PubMed
-
- Kato H, Ichinose Y, Ohta M, et al: A randomized trial of adjuvant chemotherapy with Uracil-Tegafur for adenocarcinoma of the lung. N Engl J Med 350:1713-1721, 2004 - PubMed
-
- Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N Engl J Med 352:2589-2597, 2005 - PubMed
-
- Strauss GM, Herndon JE, Maddaus MA, et al: Randomized Clinical Trial of adjuvant chemotherapy with paclitaxel and carboplatin following resection in Stage IB non–small-cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) Protocol 9633. J Clin Oncol 22:621s, 2004. (suppl; abstr 7019)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

