Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10
- PMID: 18809617
- PMCID: PMC2652099
- DOI: 10.1200/JCO.2007.12.6094
Quality-of-life outcomes for adjuvant chemotherapy in early-stage non-small-cell lung cancer: results from a randomized trial, JBR.10
Abstract
Purpose: Adjuvant chemotherapy for early stage non-small-cell lung cancer (NSCLC) is now the standard of care, but there is little information regarding its impact on quality of life (QOL). We report the QOL results of JBR.10, a North American, intergroup, randomized trial of adjuvant cisplatin and vinorelbine compared with observation in patients who have completely resected, stages IB to II NSCLC.
Patients and methods: QOL was assessed with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and a trial-specific checklist at baseline and at weeks 5 and 9 for those who received chemotherapy and at follow-up months 3, 6, 9, 12, 18, 24, 30 and 36. A 10-point change in QOL scores from baseline was considered clinically significant.
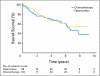
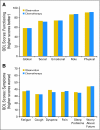
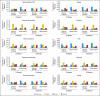
Results: Four hundred eighty-two patients were randomly assigned on JBR.10. A total of 173 patients (82% of the expected) in the observation arm and 186 (85% of expected) in the chemotherapy arm completed baseline QOL assessments. The two groups were comparable, with low global QOL scores and significant symptom burden, especially pain and fatigue, after thoracotomy. Changes in QOL during chemotherapy were relatively modest; fatigue, nausea, and vomiting worsened, but there was a reduction in pain and no change in global QOL. Patients in the observation arm showed considerable improvements in QOL by 3 months. QOL, except for symptoms of sensory neuropathy and hearing loss, in those treated with chemotherapy returned to baseline by 9 months.
Conclusion: The findings of this trial indicate that the negative effects of adjuvant chemotherapy on QOL appear to be temporary, and that improvements (with a return to baseline function) are likely in most patients.
Figures


Comment in
-
Missing patient-reported outcome data in an adjuvant lung cancer study.J Clin Oncol. 2008 Nov 1;26(31):5018-9. doi: 10.1200/JCO.2008.18.2154. Epub 2008 Sep 22. J Clin Oncol. 2008. PMID: 18809601 No abstract available.
References
-
- Jemal A, Murray T, Ward E, et al: Cancer statistics, 2005. CA Cancer J Clin 55:10-30, 2005 - PubMed
-
- Mountain C: Revisions in the International System for Staging Lung Cancer. Chest 111:1710-1717, 1997 - PubMed
-
- Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N Engl J Med 352:2589-2597, 2005 - PubMed
-
- Arriagada R, Bergman B, Dunant A, et al: Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer. N Engl J Med 350:351-360, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

