Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice
- PMID: 18809660
- PMCID: PMC2583566
- DOI: 10.1128/IAI.00874-08
Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice
Abstract
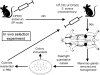
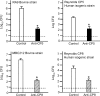
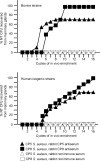
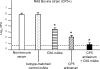
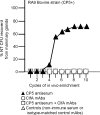
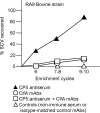
The pathogenesis of Staphylococcus aureus infections is influenced by multiple virulence factors that are expressed under variable conditions, and this has complicated the design of an effective vaccine. Clinical trials that targeted the capsule or clumping factor A (ClfA) failed to protect the recipients against staphylococcal infections. We passively immunized lactating mice with rabbit antibodies to S. aureus capsular polysaccharide (CP) serotype 5 (CP5) or CP8 or with monoclonal antibodies to ClfA. Mice immunized with antibodies to CP5 or CP8 or with ClfA had significantly reduced tissue bacterial burdens 4 days after intramammary challenge with encapsulated S. aureus strains. After several passages in mice passively immunized with CP-specific antiserum, increasing numbers of stable unencapsulated variants of S. aureus were cultured from the infected mammary glands. Greater numbers of these unencapsulated S. aureus variants than of the corresponding encapsulated parental strains were internalized in vitro in MAC-T bovine cells. Furthermore, small-colony variants (SCVs) were recovered from the infected mammary glands after several passages in mice passively immunized with CP-specific antiserum. A combination of antibodies effectively sterilized mammary glands in a significant number of passively immunized mice. More importantly, passive immunization with antibodies to both CP and ClfA fully inhibited the emergence of unencapsulated "escape mutants" and significantly reduced the appearance of SCVs. A vaccine formulation comprising CP conjugates plus a surface-associated protein adhesin may be more effective than either antigen alone for prevention of S. aureus infections.
Figures

Similar articles
-
Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo.Virulence. 2017 Aug 18;8(6):859-874. doi: 10.1080/21505594.2016.1270494. Epub 2016 Dec 9. Virulence. 2017. PMID: 27936346 Free PMC article.
-
Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice.Infect Immun. 2005 Dec;73(12):7932-7. doi: 10.1128/IAI.73.12.7932-7937.2005. Infect Immun. 2005. PMID: 16299284 Free PMC article.
-
Preclinical Efficacy of Clumping Factor A in Prevention of Staphylococcus aureus Infection.mBio. 2016 Feb 2;7(1):e02232-15. doi: 10.1128/mBio.02232-15. mBio. 2016. PMID: 26838725 Free PMC article.
-
Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus.J Med Microbiol. 1994 Feb;40(2):79-89. doi: 10.1099/00222615-40-2-79. J Med Microbiol. 1994. PMID: 8107066 Review.
-
Vaccine potential of poly-1-6 beta-D-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis.J Biotechnol. 2000 Sep 29;83(1-2):37-44. doi: 10.1016/s0168-1656(00)00296-0. J Biotechnol. 2000. PMID: 11000458 Review.
Cited by
-
Use of a human-like low-grade bacteremia model of experimental endocarditis to study the role of Staphylococcus aureus adhesins and platelet aggregation in early endocarditis.Infect Immun. 2013 Mar;81(3):697-703. doi: 10.1128/IAI.01030-12. Epub 2012 Dec 17. Infect Immun. 2013. PMID: 23250949 Free PMC article.
-
Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo.Virulence. 2017 Aug 18;8(6):859-874. doi: 10.1080/21505594.2016.1270494. Epub 2016 Dec 9. Virulence. 2017. PMID: 27936346 Free PMC article.
-
Evaluation of serotypes 5 and 8 capsular polysaccharides in protection against Staphylococcus aureus in murine models of infection.Hum Vaccin Immunother. 2017 Jul 3;13(7):1609-1614. doi: 10.1080/21645515.2017.1304334. Epub 2017 Apr 19. Hum Vaccin Immunother. 2017. PMID: 28422567 Free PMC article.
-
Immunorelevant proteins for the diagnosis of bovine staphylococcal mastitis.World J Microbiol Biotechnol. 2013 Jul;29(7):1155-60. doi: 10.1007/s11274-013-1274-8. Epub 2013 Feb 6. World J Microbiol Biotechnol. 2013. PMID: 23386318
-
Evaluation of clumping factor A binding region A in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice.Clin Vaccine Immunol. 2010 Nov;17(11):1746-52. doi: 10.1128/CVI.00162-10. Epub 2010 Sep 8. Clin Vaccine Immunol. 2010. PMID: 20826613 Free PMC article.
References
-
- Brouillette, E., A. Martinez, B. J. Boyll, N. E. Allen, and F. Malouin. 2004. Persistence of a Staphylococcus aureus small-colony variant under antibiotic pressure in vivo. FEMS Immunol. Med. Microbiol. 4135-41. - PubMed
-
- Buzzola, F. R., L. P. Alvarez, L. P. N. Tuchscherr, M. S. Barbagelata, S. M. Lattar, L. Calvinho, and D. O. Sordelli. 2007. Differential abilities of capsulated and noncapsulated Staphylococcus aureus isolates from diverse agr groups to invade mammary epithelial cells. Infect. Immun. 75886-891. - PMC - PubMed
-
- CDC. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 51565-567. - PubMed
-
- Cocchiaro, J. L., M. I. Gómez, A. Risley, R. Solinga, D. O. Sordelli, and J. C. Lee. 2006. Molecular characterization of the capsule locus from nontypeable Staphylococcus aureus. Mol. Microbiol. 59948-960. - PubMed
-
- DeJonge, M., D. Burchfield, B. Bloom, M. Duenas, W. Walker, M. Polak, E. Jung, D. Millard, R. Schelonka, F. Eyal, A. Morris, B. Kapik, D. Roberson, K. Kesler, J. Patti, and S. Hetherington. 2007. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J. Pediatr. 151260-265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

