Snake phospholipase A2 neurotoxins enter neurons, bind specifically to mitochondria, and open their transition pores
- PMID: 18809685
- PMCID: PMC2662223
- DOI: 10.1074/jbc.M803243200
Snake phospholipase A2 neurotoxins enter neurons, bind specifically to mitochondria, and open their transition pores
Abstract
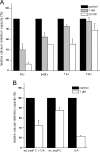
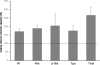
Snake presynaptic neurotoxins with phospholipase A(2) activity are potent inducers of paralysis through inhibition of the neuromuscular junction. These neurotoxins were recently shown to induce exocytosis of synaptic vesicles following the production of lysophospholipids and fatty acids and a sustained influx of Ca(2+) from the medium. Here, we show that these toxins are able to penetrate spinal cord motor neurons and cerebellar granule neurons and selectively bind to mitochondria. As a result of this interaction, mitochondria depolarize and undergo a profound shape change from elongated and spaghetti-like to round and swollen. We show that snake presynaptic phospholipase A(2) neurotoxins facilitate opening of the mitochondrial permeability transition pore, an inner membrane high-conductance channel. The relative potency of the snake neurotoxins was similar for the permeability transition pore opening and for the phospholipid hydrolysis activities, suggesting a causal relationship, which is also supported by the effect of phospholipid hydrolysis products, lysophospholipids and fatty acids, on mitochondrial pore opening. These findings contribute to define the cellular events that lead to intoxication of nerve terminals by these snake neurotoxins and suggest that mitochondrial impairment is an important determinant of their toxicity.
Figures





Similar articles
-
Calcium influx and mitochondrial alterations at synapses exposed to snake neurotoxins or their phospholipid hydrolysis products.J Biol Chem. 2007 Apr 13;282(15):11238-45. doi: 10.1074/jbc.M610176200. Epub 2007 Feb 20. J Biol Chem. 2007. PMID: 17311918
-
Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures.Science. 2005 Dec 9;310(5754):1678-80. doi: 10.1126/science.1120640. Science. 2005. PMID: 16339444
-
Calcium overload in nerve terminals of cultured neurons intoxicated by alpha-latrotoxin and snake PLA2 neurotoxins.Toxicon. 2009 Aug;54(2):138-44. doi: 10.1016/j.toxicon.2009.03.025. Epub 2009 Mar 31. Toxicon. 2009. PMID: 19341756
-
Different mechanism of blockade of neuroexocytosis by presynaptic neurotoxins.Toxicol Lett. 2004 Apr 1;149(1-3):91-101. doi: 10.1016/j.toxlet.2003.12.023. Toxicol Lett. 2004. PMID: 15093253 Review.
-
Different mechanisms of inhibition of nerve terminals by botulinum and snake presynaptic neurotoxins.Toxicon. 2009 Oct;54(5):561-4. doi: 10.1016/j.toxicon.2008.12.012. Epub 2008 Dec 14. Toxicon. 2009. PMID: 19111566 Review.
Cited by
-
Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming.Toxins (Basel). 2017 Apr 19;9(4):143. doi: 10.3390/toxins9040143. Toxins (Basel). 2017. PMID: 28422078 Free PMC article. Review.
-
Enzymatic activity and brine shrimp lethality of venom from the large brown spitting cobra (Naja ashei) and its neutralization by antivenom.BMC Res Notes. 2020 Jul 6;13(1):325. doi: 10.1186/s13104-020-05167-2. BMC Res Notes. 2020. PMID: 32631407 Free PMC article.
-
Agonists of melatonin receptors strongly promote the functional recovery from the neuroparalysis induced by neurotoxic snakes.PLoS Negl Trop Dis. 2024 Jan 8;18(1):e0011825. doi: 10.1371/journal.pntd.0011825. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38190386 Free PMC article.
-
Neurotoxicity in snakebite--the limits of our knowledge.PLoS Negl Trop Dis. 2013 Oct 10;7(10):e2302. doi: 10.1371/journal.pntd.0002302. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24130909 Free PMC article. Review.
-
The inhibitory effect of Camellia sinensis extracts against the neuromuscular blockade of Crotalus durissus terrificus venom.J Venom Res. 2010 Sep 30;1:1-7. J Venom Res. 2010. PMID: 21544176 Free PMC article.
References
-
- Rossetto, O., Morbiato, L., Caccin, P., Rigoni, M., and Montecucco, C. (2006) J. Neurochem. 97 1534-1545 - PubMed
-
- Connolly, S., and Warrell, D. A. (1995) Ann. Neurol. 38 916-920 - PubMed
-
- Prasarnpun, S., Walsh, J., Awad, S. S., and Harris, J. B. (2005) Brain 128 2987-2996 - PubMed
-
- Rossetto, O., and Montecucco, C. (2008) Handb. Exp. Pharmacol. 184 129-170 - PubMed
-
- Rigoni, M., Schiavo, G., Weston, A. E., Caccin, P., Allegroni, F., Pennuto, M., Valtorta, F., Montecucco, C., and Rossetto, O. (2004) J. Cell Sci. 117 3561-3570 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

