The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1
- PMID: 18809728
- PMCID: PMC2542481
- DOI: 10.1083/jcb.200801121
The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1
Abstract
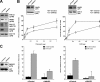
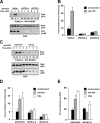
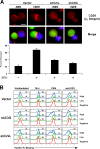
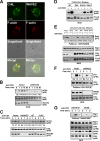
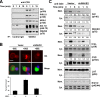
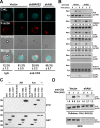
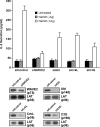
WAVE2 regulates T cell receptor (TCR)-stimulated actin cytoskeletal dynamics leading to both integrin clustering and affinity maturation. Although WAVE2 mediates integrin affinity maturation by recruiting vinculin and talin to the immunological synapse in an Arp2/3-dependent manner, the mechanism by which it regulates integrin clustering is unclear. We show that the Abl tyrosine kinase associates with the WAVE2 complex and TCR ligation induces WAVE2-dependent membrane recruitment of Abl. Furthermore, we show that WAVE2 regulates TCR-mediated activation of the integrin regulatory guanosine triphosphatase Rap1 via the recruitment and activation of the CrkL-C3G exchange complex. Moreover, we demonstrate that although Abl does not regulate the recruitment of CrkL-C3G into the membrane, it does affect the tyrosine phosphorylation of C3G, which is required for its guanine nucleotide exchange factor activity toward Rap1. This signaling node regulates not only TCR-stimulated integrin clustering but also affinity maturation. These findings identify a previously unknown mechanism by which the WAVE2 complex regulates TCR signaling to Rap1 and integrin activation.
Figures


References
-
- Anton van der Merwe, P., S.J. Davis, A.S. Shaw, and M.L. Dustin. 2000. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12:5–21. - PubMed
-
- Arai, A., Y. Nosaka, H. Kohsaka, N. Miyasaka, and O. Miura. 1999. CrkL activates integrin-mediated hematopoietic cell adhesion through the guanine nucleotide exchange factor C3G. Blood. 93:3713–3722. - PubMed
-
- Billadeau, D.D., J.C. Nolz, and T.S. Gomez. 2007. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7:131–143. - PubMed
-
- Bos, J.L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123–128. - PubMed
-
- Cannon, J.L., C.M. Labno, G. Bosco, A. Seth, M.H. McGavin, K.A. Siminovitch, M.K. Rosen, and J.K. Burkhardt. 2001. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 15:249–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

