New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity
- PMID: 18809908
- PMCID: PMC2547466
- DOI: 10.1073/pnas.0807374105
New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity
Abstract
Co-expression and gene linkage have hampered elucidating the physiological relevance of cadherins in mammalian tissues. Here, we combine conditional gene ablation and transgenic RNA interference to uncover new roles for E- and P-cadherins in epidermal sheet formation in vitro and maintenance of epidermal integrity in vivo. By devising skin-specific RNAi technology, we demonstrate that cadherin inhibition in vivo impairs junction formation and intercellular adhesion and increases apoptosis. These defects compromise epidermal barrier function and tissue integrity. In vitro, with only E-cadherin missing, epidermal sheet formation is delayed, but when both cadherins are suppressed, defects extend to adherens junctions, desmosomes, tight junctions and cortical actin dynamics. Using different rescue strategies, we show that cadherin level rather than subtype is critical. Finally, by comparing conditional loss-of-function studies of epidermal catenins and cadherins, we dissect cadherin-dependent and independent roles of adherens junction components in tissue physiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

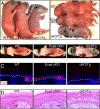


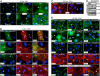

Comment in
-
Dissecting the role of cadherin-catenin proteins in mammalian epidermis.Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15225-6. doi: 10.1073/pnas.0808458105. Epub 2008 Oct 1. Proc Natl Acad Sci U S A. 2008. PMID: 18832145 Free PMC article. No abstract available.
References
-
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. - PubMed
-
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. - PubMed
-
- Patel SD, et al. Type II cadherin ectodomain structures: Implications for classical cadherin specificity. Cell. 2006;124:1255–1268. - PubMed
-
- Halbleib JM, Nelson WJ. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

