CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS
- PMID: 18809917
- PMCID: PMC2547419
- DOI: 10.1073/pnas.0807419105
CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS
Abstract
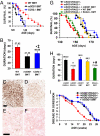
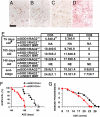
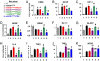
Neuroinflammation, marked by gliosis and infiltrating T cells, is a prominent pathological feature in diverse models of dominantly inherited neurodegenerative diseases. Recent evidence derived from transgenic mice ubiquitously overexpressing mutant Cu(2+)/Zn(2+) superoxide dismutase (mSOD1), a chronic neurodegenerative model of inherited amyotrophic lateral sclerosis (ALS), indicates that glia with either a lack of or reduction in mSOD1 expression enhance motoneuron protection and slow disease progression. However, the contribution of T cells that are present at sites of motoneuron injury in mSOD1 transgenic mice is not known. Here we show that when mSOD1 mice were bred with mice lacking functional T cells or CD4+ T cells, motoneuron disease was accelerated, accompanied by unexpected attenuated morphological markers of gliosis, increased mRNA levels for proinflammatory cytokines and NOX2, and decreased levels of trophic factors and glial glutamate transporters. Bone marrow transplants reconstituted mice with T cells, prolonged survival, suppressed cytotoxicity, and restored glial activation. These results demonstrate for the first time in a model of chronic neurodegeneration that morphological activation of microglia and astroglia does not predict glial function, and that the presence of CD4+ T cells provides supportive neuroprotection by modulating the trophic/cytotoxic balance of glia. These glial/T-cell interactions establish a novel target for therapeutic intervention in ALS and possibly other neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

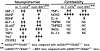
References
-
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. - PubMed
-
- Gurney M, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
-
- Boillée S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

