The mammary microenvironment alters the differentiation repertoire of neural stem cells
- PMID: 18809919
- PMCID: PMC2567463
- DOI: 10.1073/pnas.0803214105
The mammary microenvironment alters the differentiation repertoire of neural stem cells
Abstract
A fundamental issue in stem cell biology is whether adult somatic stem cells are capable of accessing alternate tissue sites and continue functioning as stem cells in the new microenvironment. To address this issue relative to neurogenic stem cells in the mouse mammary gland microenvironment, we mixed wild-type mammary epithelial cells (MECs) with bona fide neural stem cells (NSCs) isolated from WAP-Cre/Rosa26R mice and inoculated them into cleared fat pads of immunocompromised females. Hosts were bred 6-8 weeks later and examined postinvolution. This allowed for mammary tissue growth, transient activation of the WAP-Cre gene, recombination, and constitutive expression of LacZ. The NSCs and their progeny contributed to mammary epithelial growth during ductal morphogenesis, and the Rosa26-LacZ reporter gene was activated by WAP-Cre expression during pregnancy. Some NSC-derived LacZ(+) cells expressed mammary-specific functions, including milk protein synthesis, whereas others adopted myoepithelial cell fates. Thus, NSCs and their progeny enter mammary epithelium-specific niches and adopt the function of similarly endowed mammary cells. This result supports the conclusion that tissue-specific signals emanating from the stroma and from the differentiated somatic cells of the mouse mammary gland can redirect the NSCs to produce cellular progeny committed to MEC fates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


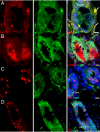
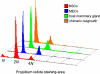

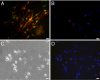
Comment in
-
Reprogramming stem cells is a microenvironmental task.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15637-8. doi: 10.1073/pnas.0808457105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843110 Free PMC article. Review. No abstract available.
References
-
- Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. - PubMed
-
- Androutsellis-Theotokis A, et al. In: Methods in Molecular Biology. Weiner LP, editor. Vol 438. Totowa, NJ: Humana; 2008. pp. 31–38. - PubMed
-
- Williams JM, Daniel CW. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. - PubMed
-
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

