Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation
- PMID: 18809926
- PMCID: PMC2567506
- DOI: 10.1073/pnas.0806486105
Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation
Abstract
The S4 transmembrane segments of voltage-gated ion channels move outward on depolarization, initiating a conformational change that opens the pore, but the mechanism of S4 movement is unresolved. One structural model predicts sequential formation of ion pairs between the S4 gating charges and negative charges in neighboring S2 and S3 transmembrane segments during gating. Here, we show that paired cysteine substitutions for the third gating charge (R3) in S4 and D60 in S2 of the bacterial sodium channel NaChBac form a disulfide bond during activation, thus "locking" the S4 segment and inducing slow inactivation of the channel. Disulfide locking closely followed the kinetics and voltage dependence of activation and was reversed by hyperpolarization. Activation of D60C:R3C channels is favored compared with single cysteine mutants, and mutant cycle analysis revealed strong free-energy coupling between these residues, further supporting interaction of R3 and D60 during gating. Our results demonstrate voltage-dependent formation of an ion pair during activation of the voltage sensor in real time and suggest that this interaction catalyzes S4 movement and channel activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


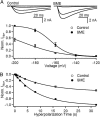
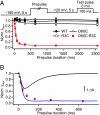

References
-
- Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. - PubMed
-
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. - PubMed
-
- Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

