Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics
- PMID: 18812550
- PMCID: PMC2556704
- DOI: 10.1093/jnci/djn309
Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics
Abstract
Background: Understanding how tumor response is related to relapse risk would help clinicians make decisions about additional treatment options for patients who have received neoadjuvant endocrine treatment for estrogen receptor-positive (ER+) breast cancer.
Methods: Tumors from 228 postmenopausal women with confirmed ER+ stage 2 and 3 breast cancers in the P024 neoadjuvant endocrine therapy trial, which compared letrozole and tamoxifen for 4 months before surgery, were analyzed for posttreatment ER status, Ki67 proliferation index, histological grade, pathological tumor size, node status, and treatment response. Cox proportional hazards were used to identify factors associated with relapse-free survival (RFS) and breast cancer-specific survival (BCSS) in 158 women. A preoperative endocrine prognostic index (PEPI) for RFS was developed from these data and validated in an independent study of 203 postmenopausal women in the IMPACT trial, which compared treatment with anastrozole, tamoxifen, or the combination 3 months before surgery. Statistical tests were two-sided.
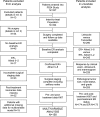
Results: Median follow-up in P024 was 61.2 months. Patients with confirmed baseline ER+ clinical stage 2 and 3 tumors that were downstaged to stage 1 or 0 at surgery had 100% RFS (compared with higher stages, P < .001). Multivariable testing of posttreatment tumor characteristics revealed that pathological tumor size, node status, Ki67 level, and ER status were independently associated with both RFS and BCSS. The PEPI model based on these factors predicted RFS in the IMPACT trial (P = .002).
Conclusions: Breast cancer patients with pathological stage 1 or 0 disease after neoadjuvant endocrine therapy and a low-risk biomarker profile in the surgical specimen (PEPI score 0) have an extremely low risk of relapse and are therefore unlikely to benefit from adjuvant chemotherapy.
Figures


References
-
- Bast RC, Jr, Hortobagyi GN. Individualized care for patients with cancer—a work in progress. N Engl J Med. 2004;351(27):2865–2867. - PubMed
-
- Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25(19):2650–2655. - PubMed
-
- Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12(11):1527–1532. - PubMed
-
- Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808–3816. - PubMed
-
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

