Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation
- PMID: 18812596
- PMCID: PMC2636918
- DOI: 10.1194/jlr.M700505-JLR200
Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation
Abstract
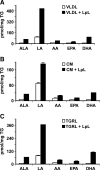
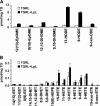
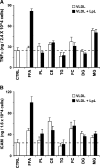
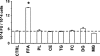
Triglyceride-rich lipoprotein (TGRL) lipolysis products provide a pro-inflammatory stimulus that can alter endothelial barrier function. To probe the mechanism of this lipolysis-induced event, we evaluated the pro-inflammatory potential of lipid classes derived from human postprandial TGRL by lipoprotein lipase (LpL). Incubation of TGRL with LpL for 30 min increased the saturated and unsaturated FFA content of the incubation solutions significantly. Furthermore, concentrations of the hydroxylated linoleates 9-hydroxy ocatadecadienoic acid (9-HODE) and 13-HODE were elevated by LpL lipolysis, more than other measured oxylipids. The FFA fractions elicited pro-inflammatory responses inducing TNFalpha and intracellular adhesion molecule expression and reactive oxygen species (ROS) production in human aortic endothelial cells (HAECs). The FFA-mediated increase in ROS was blocked by both the cytochrome P450 2C9 inhibitor sulfaphenazole and NADPH oxidase inhibitors. Compared with linoleate, 13-HODE was found to be a more potent inducer of ROS production in HAECs, an activity that was insensitive to both NADPH oxidase and cytochrome P450 inhibitors. Therefore, although the oxidative metabolism of FFA in endothelial cells can produce inflammatory responses, TGRL lipolysis can also release preformed mediators of oxidative stress (e.g., HODEs) that may influence endothelial cell function in vivo by stimulating intracellular ROS production.
Figures
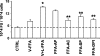
References
-
- Vogel R. A., M. C. Corretti, and G. D. Plotnick. 1997. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 79 350–354. - PubMed
-
- Karpe F. 1997. Postprandial lipid metabolism in relation to coronary heart disease. Proc. Nutr. Soc. 56 671–678. - PubMed
-
- Zilversmit D. B. 1979. Atherogenesis: a postprandial phenomenon. Circulation. 60 473–485. - PubMed
-
- Zilversmit D. B. 1995. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin. Chem. 41 153–158. - PubMed
-
- Lundman P., M. J. Eriksson, M. Stuhlinger, J. P. Cooke, A. Hamsten, and P. Tornvall. 2001. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J. Am. Coll. Cardiol. 38 111–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

