Isolation of human monoclonal antibodies by mammalian cell display
- PMID: 18812621
- PMCID: PMC2567231
- DOI: 10.1073/pnas.0805942105
Isolation of human monoclonal antibodies by mammalian cell display
Abstract
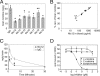
Due to their low immunogenicity in patients, humanized or fully human mAbs are becoming increasingly important for the treatment of a growing number of diseases, including cancer, infections, and immune disorders. Here, we describe a technology allowing for the rapid isolation of fully human mAbs. In contrast to previously described methods, B cells specific for an antigen of interest are directly isolated from peripheral blood mononuclear cells (PBMC) of human donors. Recombinant, antigen-specific single-chain Fv (scFv) libraries are generated from this pool of B cells and screened by mammalian cell surface display by using a Sindbis virus expression system. This method allows isolating antigen-specific antibodies by a single round of FACS. The variable regions (VRs) of the heavy chains (HCs) and light chains (LCs) are isolated from positive clones and recombinant fully human antibodies produced as whole IgG or Fab fragments. In this manner, several hypermutated high-affinity antibodies binding the Qbeta virus like particle (VLP), a model viral antigen, as well as antibodies specific for nicotine were isolated. All antibodies showed high expression levels in cell culture. The human nicotine-specific mAbs were validated preclinically in a mouse model. Thus, the technology presented here allows for rapid isolation of high-affinity, fully human antibodies with therapeutic potential from human volunteers.
Conflict of interest statement
Conflict of interest statement: All authors are employees and owners of stock options of Cytos Biotechnology AG, and therefore declare potential competing interest.
Figures


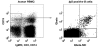
References
-
- Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. - PubMed
-
- Miller RA, Oseroff AR, Stratte PT, Levy R. Monoclonal antibody therapeutic trials in seven patients with T-cell lymphoma. Blood. 1983;62:988–995. - PubMed
-
- Ratner B. Allergy, Anaphylaxis and Immunotherapy, Basic Principles and Practice. Baltimore: Williams and Wilkins; 1943.
-
- Foote J, Winter G. Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol. 1992;224:487–499. - PubMed
-
- Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

