Effects of the renal medullary pH and ionic environment on vasopressin binding and signaling
- PMID: 18813286
- PMCID: PMC3730289
- DOI: 10.1038/ki.2008.412
Effects of the renal medullary pH and ionic environment on vasopressin binding and signaling
Abstract
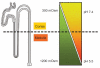
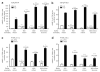
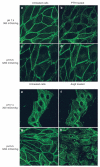
The kidney has a cortico-medullary interstitial gradient of decreasing pH and increasing concentrations of sodium chloride and urea, but the influence of these gradients on receptor signaling is largely unknown. Here, we measured G-protein coupled receptor function in LLC-PK1 cells acutely exposed to conditions mimicking different kidney regions. Signaling through the parathyroid hormone receptor, normally expressed in the cortex, was greatly reduced at an acidic pH similar to that of the inner medulla. Parathyroid hormone receptor, tagged with green fluorescent protein, showed no ligand-induced internalization. In contrast, under both acidic and hyperosmotic conditions, vasopressin increased intracellular cAMP, and upon binding to its type 2 receptor (V2R) was internalized and degraded. Dose-displacement binding assays with selective vasopressin/oxytocin receptor ligands under inner medullary conditions indicated a shift in the V2R pharmacological profile. Oxytocin did not bind to the V2R, as it does under normal conditions and the vasopressin type 1 receptor (V1R) had reduced affinity for vasopressin compared to the V2R in low pH and high osmolality. We suggest that the cortico-medullary gradient causes a receptor-specific selectivity in ligand binding that is of functional significance to the kidney. While the gradient is important for urinary concentration, it may also play a substantial role in fine-tuning of the vasopressin response through the V2R.
Figures

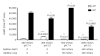


References
-
- Birnbaumer M, Seibold A, Gilbert S, et al. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357:333–335. - PubMed
-
- Lolait SJ, O'Carroll AM, McBride OW, et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. - PubMed
-
- Morel A, O'Carroll AM, Brownstein MJ, et al. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. - PubMed
-
- Sugimoto T, Saito M, Mochizuki S, et al. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. - PubMed
-
- Calo G, Rizzi A, Traina L, et al. Pharmacological characterization of a vasopressin V1 receptor in the isolated human gastric artery. Life Sci. 1997;60:PL63–PL68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

