Phagosome maturation: going through the acid test
- PMID: 18813294
- PMCID: PMC2908392
- DOI: 10.1038/nrm2515
Phagosome maturation: going through the acid test
Abstract
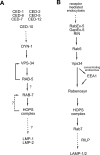
Phagosome maturation is the process by which internalized particles (such as bacteria and apoptotic cells) are trafficked into a series of increasingly acidified membrane-bound structures, leading to particle degradation. The characterization of the phagosomal proteome and studies in model organisms and mammals have led to the identification of numerous candidate proteins that cooperate to control the maturation of phagosomes containing different particles. A subset of these candidate proteins makes up the first pathway to be identified for the maturation of apoptotic cell-containing phagosomes. This suggests that a machinery that is distinct from receptor-mediated endocytosis is used in phagosome maturation.
Figures

References
-
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–102. - PubMed
-
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. - PubMed
-
- Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–103. - PubMed
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. - PubMed
-
- Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

