Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search
- PMID: 18815101
- PMCID: PMC2614384
- DOI: 10.1109/TMI.2008.923966
Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search
Abstract
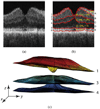
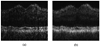
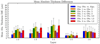
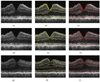
Current techniques for segmenting macular optical coherence tomography (OCT) images have been 2-D in nature. Furthermore, commercially available OCT systems have only focused on segmenting a single layer of the retina, even though each intraretinal layer may be affected differently by disease. We report an automated approach for segmenting (anisotropic) 3-D macular OCT scans into five layers. Each macular OCT dataset consisted of six linear radial scans centered at the fovea. The six surfaces defining the five layers were identified on each 3-D composite image by transforming the segmentation task into that of finding a minimum-cost closed set in a geometric graph constructed from edge/regional information and a priori determined surface smoothness and interaction constraints. The method was applied to the macular OCT scans of 12 patients (24 3-D composite image datasets) with unilateral anterior ischemic optic neuropathy (AION). Using the average of three experts' tracings as a reference standard resulted in an overall mean unsigned border positioning error of 6.1 +/- 2.9 microm, a result comparable to the interobserver variability (6.9 +/- 3.3 microm). Our quantitative analysis of the automated segmentation results from AION subject data revealed that the inner retinal layer thickness for the affected eye was 24.1 microm (21%) smaller on average than for the unaffected eye (p < 0.001), supporting the need for segmenting the layers separately.
Figures







References
-
- Koozekanani D, Boyer K, Roberts C. Retinal thickness measurements in optical coherence tomography using a Markov boundary model; Proc. IEEE Conf. Comput. Vis. Pattern Recognit. (CVPR); 2000. Jun. pp. 363–370.
-
- Koozekanani D, Boyer K, Roberts C. Retinal thickness measurements from optical coherence tomography using a Markov boundary model. IEEE Trans. Med. Imag. 2001 Sep.vol. 20(no. 9):900–916. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

