Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration
- PMID: 18815260
- PMCID: PMC6671223
- DOI: 10.1523/JNEUROSCI.0458-08.2008
Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration
Abstract
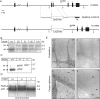
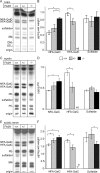
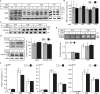
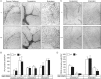
Sphingolipids containing 2-hydroxylated fatty acids are among the most abundant lipid components of the myelin sheath and therefore are thought to play an important role in formation and function of myelin. To prove this hypothesis, we generated mice lacking a functional fatty acid 2-hydroxylase (FA2H) gene. FA2H-deficient (FA2H(-/-)) mice lacked 2-hydroxylated sphingolipids in the brain and in peripheral nerves. In contrast, nonhydroxylated galactosylceramide was increased in FA2H(-/-) mice. However, oligodendrocyte differentiation examined by in situ hybridization with cRNA probes for proteolipid protein and PDGFalpha receptor and the time course of myelin formation were not altered in FA2H(-/-) mice compared with wild-type littermates. Nerve conduction velocity measurements of sciatic nerves revealed no significant differences between FA2H(-/-) and wild-type mice. Moreover, myelin of FA2H(-/-) mice up to 5 months of age appeared normal at the ultrastructural level, in the CNS and peripheral nervous system. Myelin thickness and g-ratios were normal in FA2H(-/-) mice. Aged (18-month-old) FA2H(-/-) mice, however, exhibited scattered axonal and myelin sheath degeneration in the spinal cord and an even more pronounced loss of stainability of myelin sheaths in sciatic nerves. These results show that structurally and functionally normal myelin can be formed in the absence of 2-hydroxylated sphingolipids but that its long-term maintenance is strikingly impaired. Because axon degeneration appear to start rather early with respect to myelin degenerations, these lipids might be required for glial support of axon function.
Figures






References
-
- Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. The human FA2H gene encodes a fatty acid 2-hydroxylase. J Biol Chem. 2004;279:48562–48568. - PubMed
-
- Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res. 2006;47:2772–2780. - PubMed
-
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. - PubMed
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
