Adhesive activity of Lu glycoproteins is regulated by interaction with spectrin
- PMID: 18815288
- PMCID: PMC2597615
- DOI: 10.1182/blood-2008-03-146068
Adhesive activity of Lu glycoproteins is regulated by interaction with spectrin
Abstract
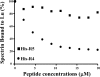
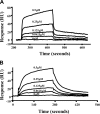
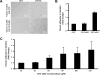
The Lutheran (Lu) and Lu(v13) blood group glycoproteins function as receptors for extracellular matrix laminins. Lu and Lu(v13) are linked to the erythrocyte cytoskeleton through a direct interaction with spectrin. However, neither the molecular basis of the interaction nor its functional consequences have previously been delineated. In the present study, we defined the binding motifs of Lu and Lu(v13) on spectrin and identified a functional role for this interaction. We found that the cytoplasmic domains of both Lu and Lu(v13) bound to repeat 4 of the alpha spectrin chain. The interaction of full-length spectrin dimer to Lu and Lu(v13) was inhibited by repeat 4 of alpha-spectrin. Further, resealing of this repeat peptide into erythrocytes led to weakened Lu-cytoskeleton interaction as demonstrated by increased detergent extractability of Lu. Importantly, disruption of the Lu-spectrin linkage was accompanied by enhanced cell adhesion to laminin. We conclude that the interaction of the Lu cytoplasmic tail with the cytoskeleton regulates its adhesive receptor function.
Figures


References
-
- El Nemer W, Gane P, Colin Y, et al. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J Biol Chem. 1998;273:16686–16693. - PubMed
-
- Hillery CA, Du MC, Montgomery RR, Scott JP. Increased adhesion of erythrocytes to components of the extracellular matrix: isolation and characterization of a red blood cell lipid that binds thrombospondin and laminin. Blood. 1996;87:4879–4886. - PubMed
-
- Lee SP, Cunningham ML, Hines PC, Joneckis CC, Orringer EP, Parise LV. Sickle cell adhesion to laminin: potential role for the alpha5 chain. Blood. 1998;92:2951–2958. - PubMed
-
- Parsons SF, Spring FA, Chasis JA, Anstee DJ. Erythroid cell adhesion molecules Lutheran and LW in health and disease. Ballieres Best Pract Res Clin Haematol. 1999:729–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

