Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C
- PMID: 18815292
- PMCID: PMC2583680
- DOI: 10.1128/JVI.01762-08
Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C
Abstract
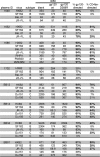
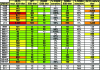
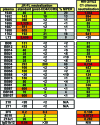
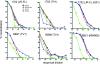
Identifying the viral epitopes targeted by broad neutralizing antibodies (NAbs) that sometimes develop in human immunodeficiency virus type 1 (HIV-1)-infected subjects should assist in the design of vaccines to elicit similar responses. Here, we investigated the activities of a panel of 24 broadly neutralizing plasmas from subtype B- and C-infected donors using a series of complementary mapping methods, focusing mostly on JR-FL as a prototype subtype B primary isolate. Adsorption with gp120 immobilized on beads revealed that an often large but variable fraction of plasma neutralization was directed to gp120 and that in some cases, neutralization was largely mediated by CD4 binding site (CD4bs) Abs. The results of a native polyacrylamide gel electrophoresis assay using JR-FL trimers further suggested that half of the subtype B and a smaller fraction of subtype C plasmas contained a significant proportion of NAbs directed to the CD4bs. Anti-gp41 neutralizing activity was detected in several plasmas of both subtypes, but in all but one case, constituted only a minor fraction of the overall neutralization activity. Assessment of the activities of the subtype B plasmas against chimeric HIV-2 viruses bearing various fragments of the membrane proximal external region (MPER) of HIV-1 gp41 revealed mixed patterns, implying that MPER neutralization was not dominated by any single specificity akin to known MPER-specific monoclonal Abs. V3 and 2G12-like NAbs appeared to make little or no contribution to JR-FL neutralization titers. Overall, we observed significant titers of anti-CD4bs NAbs in several plasmas, but approximately two-thirds of the neutralizing activity remained undefined, suggesting the existence of NAbs with specificities unlike any characterized to date.
Figures






References
-
- Bibollet-Ruche, F., L. Hui, J. M. Decker, P. A. Geopfert, B. H. Hahn, E. Delaporte, M. Peeters, S. Allen, E. Hunter, J. Robinson, P. D. Kwong, and G. M. Shaw. 2006. Detection of novel neutralizing antibody responses to the membrane proximal external region (MPER) or gp41 following infection by HIV-1 subtypes A, B, C, D, F, G, H, CRF01, CRF02, or CRF11, abstr. 110, p. 196. Keystone Symposium X6, HIV Vaccines, Keystone, CO, 27 March to 2 April.
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

