Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus
- PMID: 18815307
- PMCID: PMC2583641
- DOI: 10.1128/JVI.01634-08
Mutational analysis of cis-acting RNA signals in segment 7 of influenza A virus
Abstract
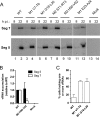
The genomic viral RNA (vRNA) segments of influenza A virus contain specific packaging signals at their termini that overlap the coding regions. To further characterize cis-acting signals in segment 7, we introduced synonymous mutations into the terminal coding regions. Mutation of codons that are normally highly conserved reduced virus growth in embryonated eggs and MDCK cells between 10- and 1,000-fold compared to that of the wild-type virus, whereas similar alterations to nonconserved codons had little effect. In all cases, the growth-impaired viruses showed defects in virion assembly and genome packaging. In eggs, nearly normal numbers of virus particles that in aggregate contained apparently equimolar quantities of the eight segments were formed, but with about fourfold less overall vRNA content than wild-type virions, suggesting that, on average, fewer than eight segments per particle were packaged. Concomitantly, the particle/PFU and segment/PFU ratios of the mutant viruses showed relative increases of up to 300-fold, with the behavior of the most defective viruses approaching that predicted for random segment packaging. Fluorescent staining of infected cells for the nucleoprotein and specific vRNAs confirmed that most mutant virus particles did not contain a full genome complement. The specific infectivity of the mutant viruses produced by MDCK cells was also reduced, but in this system, the mutations also dramatically reduced virion production. Overall, we conclude that segment 7 plays a key role in the influenza A virus genome packaging process, since mutation of as few as 4 nucleotides can dramatically inhibit infectious virus production through disruption of vRNA packaging.
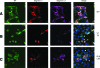
Figures



References
-
- Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 1136-42.
-
- Amorim, M. J., E. K. Read, R. M. Dalton, L. Medcalf, and P. Digard. 2007. Nuclear export of influenza A virus mRNAs requires ongoing RNA polymerase II activity. Traffic 81-11. - PubMed
-
- Bergmann, M., and T. Muster. 1996. Mutations in the nonconserved noncoding sequences of the influenza A virus segments affect viral vRNA formation. Virus Res. 4423-31. - PubMed
-
- Cheung, T. K., Y. Guan, S. S. Ng, H. Chen, C. H. Wong, J. S. Peiris, and L. L. Poon. 2005. Generation of recombinant influenza A virus without M2 ion-channel protein by introduction of a point mutation at the 5′ end of the viral intron. J. Gen. Virol. 861447-1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

