Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis
- PMID: 18815361
- PMCID: PMC2567479
- DOI: 10.1073/pnas.0803988105
Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis
Abstract
Intratumoral innate immunity can play a significant role in blocking the effective therapeutic spread of a number of oncolytic viruses (OVs). Histone deacetylase inhibitors (HDIs) are known to influence epigenetic modifications of chromatin and can blunt the cellular antiviral response. We reasoned that pretreatment of tumors with HDIs could enhance the replication and spread of OVs within malignancies. Here, we show that HDIs markedly enhance the spread of vesicular stomatitis virus (VSV) in a variety of cancer cells in vitro, in primary tumor tissue explants and in multiple animal models. This increased oncolytic activity correlated with a dampening of cellular IFN responses and augmentation of virus-induced apoptosis. These results illustrate the general utility of HDIs as chemical switches to regulate cellular innate antiviral responses and to provide controlled growth of therapeutic viruses within malignancies. HDIs could have a profoundly positive impact on the clinical implementation of OV therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


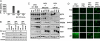

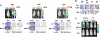
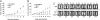
References
-
- Bell JC. Oncolytic viruses: What's next? Curr Cancer Drug Targets. 2007;7:127–131. - PubMed
-
- Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: The education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7:133–139. - PubMed
-
- Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. - PubMed
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. - PubMed
-
- Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: A new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

