In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner
- PMID: 18815375
- PMCID: PMC2567483
- DOI: 10.1073/pnas.0808248105
In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner
Abstract
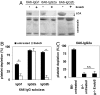
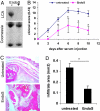
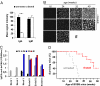
IgG antibodies are potent inducers of proinflammatory responses. During autoimmune diseases such as arthritis and systemic lupus erythematosus, IgG autoantibodies are responsible for the chronic inflammation and destruction of healthy tissues by cross-linking Fc receptors on innate immune effector cells. The sugar moiety attached to the asparagine-297 residue in the constant domain of the antibody is critical for the overall structure and function of the molecule. Removal of this sugar domain leads to the loss of the proinflammatory activity, suggesting that in vivo modulation of antibody glycosylation might be a strategy to interfere with autoimmune processes. In this work, we investigated whether removal of the majority of the IgG-associated sugar domain by endoglycosidase S (EndoS) from Streptococcus pyogenes is able to interfere with autoimmune inflammation. We demonstrate that EndoS injection efficiently removes the IgG-associated sugar domain in vivo and interferes with autoantibody-mediated proinflammatory processes in a variety of autoimmune models. Importantly, however, we observed a differential impact of EndoS-mediated sugar side chain hydrolysis on IgG activity depending on the individual IgG subclass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. - PubMed
-
- Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. - PubMed
-
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. - PubMed
-
- Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1–84. - PubMed
-
- Jefferis R, Lund J, Pound JD. IgG–Fc-mediated effector functions: Molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev. 1998;163:59–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

