New "multicolor" polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI
- PMID: 18816830
- PMCID: PMC2614370
- DOI: 10.1002/mrm.21683
New "multicolor" polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI
Abstract
An array of 33 prototype polypeptides was examined as putative contrast agents that can be distinguished from each other based on the chemical exchange saturation transfer (CEST) mechanism. These peptides were chosen based on predictions of the chemical exchange rates of exchangeable amide, amine, and hydroxyl protons that produce this contrast, and tested at 11.7T for their CEST suitability. Artificial colors were assigned to particular amino acid units (lysine, arginine, threonine, and serine) based on the separate resonance frequencies of these exchangeable protons. The magnitude of the CEST effect could be fine-tuned by altering the amino acid sequence, and these three exchangeable groups could be distinguished in an MR phantom based on their different chemical shifts ("colors"). These new diamagnetic CEST (DIACEST) agents possess a wide range of electrostatic charges, compositions, and protein stabilities in vivo, making them potentially suitable for a variety of biological applications such as designing MR reporter genes for imaging cells and distinguishing multiple targets within the same MR image.
(c) 2008 Wiley-Liss, Inc.
Figures


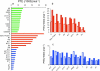
 ), PLR (35 kDa,
), PLR (35 kDa,  ) and PLT (7.6 kD,
) and PLT (7.6 kD,  ) using ω1= 2.2 μT. a) Intensity comparison of z-spectra and MTRasym spectra . b) Expanded z spectra from a. b) MTRasym spectra from a normalized to their highest intensity.
) using ω1= 2.2 μT. a) Intensity comparison of z-spectra and MTRasym spectra . b) Expanded z spectra from a. b) MTRasym spectra from a normalized to their highest intensity.
 ), gNH2 color (
), gNH2 color ( ), OH color (
), OH color ( ). b) Comparison of gNH2 color peptides, showing the gNH2 (
). b) Comparison of gNH2 color peptides, showing the gNH2 ( ), NH
), NH  , and OH (
, and OH ( ) PTE. C) Comparison OH color peptides, showing the NH (
) PTE. C) Comparison OH color peptides, showing the NH ( ), gNH2 (
), gNH2 ( ) and OH (
) and OH ( ) PTE.
) PTE.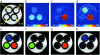
References
-
- Ward K, Aletras A, Balaban R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J Magn Reson. 2000;143:79–87. - PubMed
-
- Zhou J, vanZijl PCM. Chemical Exchange Saturation Transfer Imaging and Spectroscopy. Prog NMR Spec. 2006;48:109–136.
-
- Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. PARACEST Agents: Modulating MRI Contrast via Water Proton Exchange. Acc Chem Res. 2003;36:783–790. - PubMed
-
- McMahon MT, Zhou J, Gilad AA, Bulte JWM, van Zijl PCM. Physical Mechanism and Applications of CEST Contrast Agents. In: Bulte JWM, Modo M, editors. Molecular and Cellular MR Imaging. CRC press; Boca Raton, FL: 2007. pp. 85–100.
-
- Zhang S, Winter P, Wu K, Sherry AD. A novel europium(III)-based MRI contrast agent. J Am Chem Soc. 2001;123(7):1517–1518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

