Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis
- PMID: 18816837
- PMCID: PMC3081706
- DOI: 10.1002/dvdy.21705
Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis
Abstract
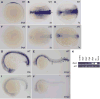
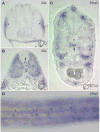
Recent studies demonstrate that lysyl oxidase cuproenzymes are critical for zebrafish notochord formation, but the molecular mechanisms of copper-dependent notochord morphogenesis are incompletely understood. We, therefore, conducted a forward genetic screen for zebrafish mutants that exhibit notochord sensitivity to lysyl oxidase inhibition, yielding a mutant with defects in notochord and vascular morphogenesis, puff daddygw1 (pfdgw1). Meiotic mapping and cloning reveal that the pfdgw1 phenotype results from disruption of the gene encoding the extracellular matrix protein fibrillin-2, and the spatiotemporal expression of fibrillin-2 is consistent with the pfdgw1 phenotype. Furthermore, each aspect of the pfdgw1 phenotype is recapitulated by morpholino knockdown of fibrillin-2. Taken together, the data reveal a genetic interaction between fibrillin-2 and the lysyl oxidases in notochord formation and demonstrate the importance of fibrillin-2 in specific early developmental processes in zebrafish.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures








References
-
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst Biol. 2006;55:539–552. - PubMed
-
- Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth CA, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278:34605–34616. - PubMed
-
- Bax DV, Mahalingam Y, Cain S, Mellody K, Freeman L, Younger K, Shuttleworth CA, Humphries MJ, Couchman JR, Kielty CM. Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J Cell Sci. 2007;120:1383–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

