Cellular localization of the activated EGFR determines its effect on cell growth in MDA-MB-468 cells
- PMID: 18817771
- PMCID: PMC2590673
- DOI: 10.1016/j.yexcr.2008.08.020
Cellular localization of the activated EGFR determines its effect on cell growth in MDA-MB-468 cells
Abstract
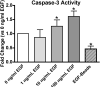
The epidermal growth factor (EGF) receptor (EGFR) is a ubiquitously expressed receptor tyrosine kinase that regulates diverse cell functions that are dependent upon cell type, the presence of downstream effectors, and receptor density. In addition to activating biochemical pathways, ligand stimulation causes the EGFR to enter the cell via clathrin-coated pits. Endocytic trafficking influences receptor signaling by controlling the duration of EGFR phosphorylation and coordinating the receptor's association with downstream effectors. To better understand the individual contributions of cell surface and cytosolic EGFRs on cell physiology, we used EGF that was conjugated to 900 nm polystyrene beads (EGF-beads). EGF-beads can stimulate the EGFR and retain the activated receptor at the plasma membrane. In MDA-MB-468 cells, a breast cancer cell line that over-expresses the EGFR, only internalized, activated EGFRs stimulate caspase-3 and induce cell death. Conversely, signaling cascades triggered from activated EGFR retained at the cell surface inhibit caspase-3 and promote cell proliferation. Thus, through endocytosis, the activated EGFR can differentially regulate cell growth in MDA-MB-468 cells.
Figures







References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB Signalling Network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. - PubMed
-
- Wiley HS, Burke PM. Regulation of Receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:557–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

