Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling
- PMID: 18818196
- PMCID: PMC2586254
- DOI: 10.1074/jbc.M804288200
Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling
Abstract
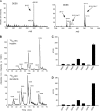
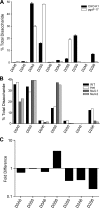
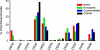
To facilitate qualitative and quantitative analysis of glycosaminoglycans, we tagged the reducing end of lyase-generated disaccharides with aniline-containing stable isotopes (12C6 and 13C6). Because different isotope tags have no effect on chromatographic retention times but can be discriminated by a mass detector, differentially isotope-tagged samples can be compared simultaneously by liquid chromatography/mass spectrometry and quantified by admixture with known amounts of standards. The technique is adaptable to all types of glycosaminoglycans, and its sensitivity is only limited by the type of mass spectrometer available. We validated the method using commercial heparin and keratan sulfate as well as heparan sulfate isolated from mutant and wild-type Chinese hamster ovary cells, and select tissues from mutant and wild-type mice. This new method provides more robust, reliable, and sensitive means of quantitative evaluation of glycosaminoglycan disaccharide compositions than existing techniques allowing us to compare the chondroitin and heparan sulfate compositions of Hydra vulgaris, Drosophila melanogaster, Caenorhabditis elegans, and mammalian cells. Our results demonstrate significant differences in glycosaminoglycan structure among these organisms that might represent evolutionarily distinct functional motifs.
Figures





References
-
- Kinoshita-Toyoda, A., Yamada, S., Haslam, S. M., Khoo, K. H., Sugiura, M., Morris, H. R., Dell, A., and Sugahara, K. (2004) Biochemistry 43 11063-11074 - PubMed
-
- Esko, J. D., and Selleck, S. B. (2002) Annu. Rev. Biochem. 71 435-471 - PubMed
-
- Toyoda, H., Kinoshita-Toyoda, A., and Selleck, S. B. (2000) J. Biol. Chem. 275 2269-2275 - PubMed
-
- Toyoda, H., Kinoshita-Toyoda, A., Fox, B., and Selleck, S. B. (2000) J. Biol. Chem. 275 21856-21861 - PubMed
-
- Yamada, S., Morimoto, H., Fujisawa, T., and Sugahara, K. (2007) Glycobiology 17 886-894 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

